* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lipid-binding proteins in rat and human kidney
Gene regulatory network wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Lipid signaling wikipedia , lookup
Secreted frizzled-related protein 1 wikipedia , lookup
Epitranscriptome wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Point mutation wikipedia , lookup
Biochemical cascade wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
Biochemistry wikipedia , lookup
Metalloprotein wikipedia , lookup
Magnesium transporter wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Gene expression wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Protein structure prediction wikipedia , lookup
Paracrine signalling wikipedia , lookup
Signal transduction wikipedia , lookup
Interactome wikipedia , lookup
Expression vector wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Western blot wikipedia , lookup
Protein–protein interaction wikipedia , lookup
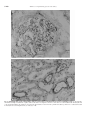
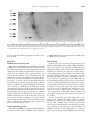
Kidney International, Vol. 56, Suppl. 71 (1999), pp. S-159–S-162 Lipid-binding proteins in rat and human kidney HIDEKI KIMURA, HIROSHI FUJII, SATORU SUZUKI, TERUO ONO, MASAAKI ARAKAWA, and FUMITAKE GEJYO Department of Clinical and Laboratory Medicine, Faculty of Medicine, Fukui Medical University, Fukui, Japan, Department of Biochemistry, Niigata University School of Medicine, Niigata, Japan, and Departments of Medicine (II), Niigata University School of Medicine, Niigata, Japan Lipid-binding proteins are expressed in the glomeruli and cultured mesangial cells of rat and human kidneys. Background. The kidney metabolizes actively lipophilic molecules. Several species of lipid-binding proteins (LBPs) have been well characterized, including fatty acid-binding proteins (FABPs), acyl-CoA binding protein (ACBP), sterol carrier protein 2 (SCP2), cellular retinol binding protein (CRBP), and phosphatidylinositol transfer protein (PITP). Methods. To clarify which LBPs are expressed in isolated rat glomeruli (RG), cultured rat mesangial cells (RMC) and human kidney, RT-PCR, immunoblot analysis and immunohistochemistry were performed. Results. Protein and mRNA expression of heart type (H-) FABP was found in RMC, but not in RG. Immunohistochemistry using antihuman H-FABP antibody revealed that an H-FABP like protein was present in the capillary wall and distal tubules of human glomeruli. Immunoblot analysis using the antibody showed that a 110-kDa protein related to H-FABP was present in human isolated glomeruli but not in any other tissues tested including blood, liver, and heart, and that the 14-kDa protein, H-FABP itself was localized in the distal tubules of human kidney. mRNA for SCP2, ACBP and PITP was detected in RG and RMC. CRBP mRNA was detected in RG but not RMC. Conclusions. A variety of lipid-binding proteins are present in rat glomeruli. In human glomeruli, a novel 110-kDa H-FABPrelated protein is localized specifically in the capillary wall. The kidney is one of the organs that most actively metabolize hydrophobic ligands. Partial segments of nephrons rapidly uptake long-chain fatty acids from blood stream, synthesize prostanoids from arachidonic acid in response to humorous factors, and modulate renal functions [1]. Diet-induced or endogenous hyperlipidemia in animal models of glomerular injury results in accelerating the progression of glomerulosclerosis [2]. A recent report of O’Donnell and coworkers [3] showed that mesangial cell proliferation is mediated by isoprenoid products of the mevalonate pathway where cholesKey words: fatty acid-binding protein, sterol carrier protein, acyl-CoA binding protein, cellular retinol binding protein, phosphatidylinositol transfer protein, glomeruli. 1999 by the International Society of Nephrology terol is endogenously synthesized. Lipid-binding proteins (LBPs), cytosolic proteins with low molecular masses (10–20 kDa), are capable of binding lipophilic molecules and are thought to play important roles in cell signaling pathways involving lipophilic molecules as well as intracellular trafficking and metabolism of lipids [4,5]. The family of LBP mainly contains fatty acid binding protein (FABP), acyl-CoA binding protein (ACBP), sterol carrier protein 2 (SCP2), cellular retinol binding protein (CRBP) and phosphatidylinositol transfer protein (PITP). Recently, we reported that heart type (H-) FABP was present in distal tubules of rat kidney [6] and that H-FABP like protein was distributed in glomerular capillary walls as well as distal tubules of human kidney [7]. However, whether other LBPs are present in glomeruli remains unknown. METHODS Normal glomeruli and tubules of rat and human kidneys were isolated by the mesh sieving technique. Rat mesangial cell (RMC) strains were isolated from male WKY rats. Cell passages 3 through 6 were used. Polyclonal antibodies against human H-FABP (HFPoA) and rat H-FABP were prepared by subcutaneous immunization of rabbit as described previously [6]. After Tricine glycine SDS-PAGE on polyacrylamide (10%) slab gels, immunoblot analysis was performed with a semidry transfer system (Bio-Rad, USA) and an ECL detection kit, (Amersham, Buckinghamshire, UK). Analysis of LBP gene expression Expression of rat H-FABP mRNA was examined by Northern blot analysis using rat H-FABP cDNA as a probe. Gene expression of other LBPs were examined with reverse transcriptase-polymerase chain reaction using specific primers. Immunohistochemistry Sections of frozen tissues (1 mm thickness) were stained by indirect immunofluorescence. Sections of paraffinembedded specimens (2 mm thickness) were examined S-159 S-160 Kimura et al: Lipid-binding proteins in the kidney Fig. 1. Immunolight micrographs of human kidney with polyclonal antibody against human heart-type fatty acid-binding protein. (A) In glomeruli, the immunostaining is found in the capillary walls but not in the mesangial area. Frozen sections were not counterstained with hematoxylin and eosin. (B) In interstitium, the staining is observed in the distal tubules. Sections from the paraffin-embedded specimens were counterstained with hematoxylin and eosin. Magnification: (A) 3400; (B) 3200. Kimura et al: Lipid-binding proteins in the kidney S-161 Fig. 2. Immunoblot analysis of different tissue homogenates probed with polyclonal antibody against human heart-type fatty acid-binding protein. Several species of human tissues were obtained from autopsy samples from a single patient. Homogenates (30 mg) of the tissues and a blood sample (30 mg) were subjected to immunoblot analysis. Lane 1, isolated glomeruli; lane 2, heart; lane 3, isolated renal tubules; lane 4, blood; lane 5, adrenal tissues; lane 6, small intestine; lane 7, lung; lane 8, spleen. by the streptavidin-biotin-complex/horseradish peroxidase method. of CRBP mRNA was detected in rat liver and RG, but not RMC (data not shown). RESULTS H-FABP and its related protein Expression of H-FABP protein and mRNA was found in RMC, but not in rat glomeruli (data not shown). Immunolight micrography of normal human kidney showed that the specific staining for H-FABP was observed exclusively in the capillary walls of glomeruli (Fig. 1A) and in the distal tubules of the interstitium (Fig. 1B). Immunoblot analysis using HFPoA showed that the immunoreactive proteins described above displayed molecular masses of 110 kDa in the glomerular homogenate and 14 kDa in the tubular homogenate (Fig. 2). Preabsorption of HFPoA by purified H-FABP at the molar ratio of antigen to antibody [50/1] diminished equally the immunoreactivities of both the 110- kDa protein in glomeruli and purified human H-FABP (data not shown), demonstrating that the two proteins shared a common antigen. This unique 110-kDa protein was not able to be immunochemically detected in homogenates from any other tissues examined, including liver, heart, skeletal muscle, adrenal gland, adipose tissue and small intestine (Fig. 2), although the 14-kDa protein was detected in heart and skeletal muscle tissue. DISCUSSION Other lipid-binding proteins Expression of mRNA for ACBP, SCP2 and PITP was detected in rat liver, RG, and RMC, while expression In the present study, we showed for the first time that mRNA for H-FABP, SCP2, ACBP, CRBP, and PITP is expressed variably in rat glomeruli or RCM. Because lipophilic molecules, such as retinoic acid, and phosphatidylinositol are known to be elements of several cell-tocell signaling pathways, these LBPs may modulate lipidmediated signal transduction in mesangial cells and glomeruli [5]. PITP is a requisite component for epidermal growth factor signaling [8], while CRBP and retinoids probably participate in the process of smooth muscle cell activation [9]. FABP may modulate the activity of peroxisome proliferator-activated receptors owing to its affinity for long-chain fatty acids, putative ligands for the receptors, and thereby may influence the gene expression of other LBPs. However, whether or not some pathogenetic relationships between FABP and other LBPs exist in progressive renal disease remains to be clarified. We also obtained the interesting finding that a 110kDa protein, immunochemically related to human H-FABP, is confined to the capillary wall in human glomeruli, while H-FABP itself, (14 kDa) was localized exclusively to the distal tubules, from the thick ascending loop of Henle to collecting ducts. The distribution of H-FABP (14 kDa) in the human kidney is consistent with our previous report that H-FABP is present in the S-162 Kimura et al: Lipid-binding proteins in the kidney distal tubules of the rat kidney [6]. In the distal tubules, mitochondrial b-oxidation of long-chain fatty acids is predominant and the high rate in prostaglandin synthesis is observed. It is intriguing that a 100-kDa ion channel in neuron called the NMDA1 receptor had about 30% homology with H-FABP in a putative fatty acid-binding domain, residue 263–393 [10], although the detailed molecular nature of the 110-kDa H-FABP-related protein remains unknown. Therefore, The H-FABP in the distal tubules may be involved in lipid metabolism such as intracellular trafficking and b-oxidation of long-chain fatty acids and prostaglandin synthesis, whereas the 110 kDa H-FABP related protein in the glomerular capillary wall may serve as a receptor that is activated by fatty acids, such as the NMDA-1 receptor. Additional studies will be required to characterize the 110 kDa protein and to clarify pathogenetic roles of LBPs in renal disease. In conclusion, many species of LBPs are present in rat glomeruli and cultured mesangial cells. In the human kidney, a novel 110-kDa H-FABP-related protein is present only in the glomerular capillary wall, while H-FABP is distributed in the distal tubules. ACKNOWLEDGMENTS This work was supported in part by a Grant-in-Aid for Scientific Research 09671159 from the Ministry of Education, Science, and Culture of Japan. The skillful technical assistance of Drs. Naofumi Imai and Hirokazu Sato is highly appreciated. Reprint requests to Hideki Kimura, M.D., Department of Clinical and Laboratory Medicine, Faculty of Medicine, Fukui Medical University, 23 Shimoaitsuki, Matsuoka, Yoshida, Fukui 910-1193, Japan. E-mail: [email protected] REFERENCES 1. Breyer MD, Badr KF: Arachidonic acid metabolites and the kidney. In: The Kidney, 5th, ed., edited by Brenner BM, Philadelphia, WB Saunders, 1996, pp. 754–788. 2. Schmitz PG, Kasiske B, O’Donnell MP, Keane WF: Lipid and progressive renal injury. Seminars Nephrol 9:354–369, 1989. 3. O’Donnell MP, Kasiske BL, Atluru D, Kim Y, Keane WF: Mesangial cell proliferation is dependent on isoprenoid products of mevalonate metabolism. J Clin Invest 91:83–87, 1993. 4. Peeters RA, Veerkamp JH, Demel RA. Biochim Biophys Acta 1002:8–13, 1989. 5. Glatz JFC, Borcher T, Spener F, Van der vusse GJ: Fatty acids in cell signaling: modulation by lipid binding proteins. Prostaglandins Leukot Essent Fatty Acids 52:121–127, 1995. 6. Kimura H, Odani S, Nishi S, Sato H, Arakawa M, Ono T: Primary structure and cellular distribution of two fatty acid binding proteins in adult rat kidneys. J Biol Chem 266:5963–5972, 1991. 7. Kimura H, Fujii H, Nishi S, Nakagawa Y, Ono T, Arakawa M: Purification and localization of two fatty acid-binding proteins in the human kidney [Abstract]. J Am Soc Nephrol 2:763, 1991. 8. Kauffmann-zeh A, Thomas GMH, Ball A, Prosser S, Cunningham E, Cockcroft S, Hsuan JJ: Requirement for phosphatidylinositol transfer protein in epidermal growth factor signaling. Sci 268:1188–1190, 1995. 9. Neuville P, Geinoz A, Benzonana G, Redard M, Gabbiani F, Ropraz P, Gabbiani G: Cellular retinol-binding protein 1 is expressed by distinct subsets of rat arterial smooth muscle cells in vitro and in vivo. Am J Pathol 150:509–521, 1997. 10. Petrou S, Ordway RW, Singer JJ, Walsh JV Jr: A putative fatty acid-binding domain of the NMDA receptor. Trends Biosci:41–42, 1993