* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Macromolecules - Van Buren Public Schools
Vectors in gene therapy wikipedia , lookup
Peptide synthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Signal transduction wikipedia , lookup
Gene expression wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Western blot wikipedia , lookup
Interactome wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Metalloprotein wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Biosynthesis wikipedia , lookup
Macromolecules
AP Biology Lecture Series
Part 2
Big Concepts and Ideas
• How are the molecules of biological systems
constructed?
• Why are particular groups of molecules
needed in biological systems?
• How do the interactions of biological
molecules lead to the emergence of life
functions?
Macromolecules
• Huge (on molecular scale)
• Accomplish all life functions
• Made predominately of a few
common atoms, repeated and in
multiple configurations
• Can be incredibly complex
• Carbohydrates, lipids*, proteins,
nucleic acids
Macromolecules
• Polymer – long molecule consisting of many
similar subunits (3 of 4 major molecules are
polymers – excludes lipids)
– Subunits linked with covalent bonds
• Monomers – the repeating subunits of
polymers; building blocks
• Building/breaking processes rely on H2O
Polymer Synthesis & Breakdown
• Term note: Synthesis = to make or form
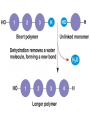
• Condensation Reactions
– Dehydration reactions (water removed)
– Bonds form between two monomers (one donates
–OH, other –H)
– Forms chain and complexity (anabolic)
– Requires energy (endergonic)
– Facilitated by enzymes
Polymer Synthesis & Breakdown
• Term note: Hydro = water; Lysis= to split
• Hydrolysis Reactions
– Break bonds between monomers using water
(H2O goes into reaction)
– Reduces complexity (catabolic)
– Released energy (exergonic)
– Facilitated by enzymes
– Allows food to be digested
Carbohydrates!
Carbohydrates
• Sugars or starches (common terms)
• Monomer = monosaccharide
– C, H, O in repeat w/1:2:1 ratio
– # of C’s varies by monomer
– Two monomer units = disaccharide
• Polymer = polysaccharide
• General use: short term energy & structure
Carbohydrates
Functional
groups
Critical for metabolic processes
Critical in DNA
Carbohydrates
• Hexose sugars are the most "famous"
monosaccharide
• Three kinds: Glucose, Galactose, & Fructose
• They are typically shown as carbon rings.
Carbohydrates
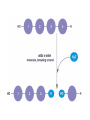
• Combine 2 monomers by dehydration
synthesis get a "disaccharide”
• Glucose + Glucose = Maltose ("Malt sugar")
• Glucose + Fructose = Sucrose ("Table sugar")
• Glucose + Galactose = Lactose ("Milk sugar")
Dehydration Synthesis…
Polysaccharides
• Massive polymers of
sugars are called
"polysaccharides”
• Two main functions in
organisms Energy
storage and structure
Polysaccharides – Energy storage
• Polysaccharides are great for short term storing of
energy.
• In plants, amylose ("starch") is the major energy
storage polysaccharide.
• Animals use glycogen for energy storage
Polysaccharides - Structure
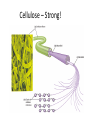
• Cellulose = major component of plant-like cell
walls.
– Most abundant organic compound on Earth!
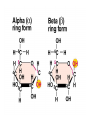
• The difference between starch and cellulose is in
the linkages between glucose units.
– Starch = alpha linked. Cellulose = beta linked
– Differ in placement of hydroxyl group
Cellulose – Strong!
Herbivores and Cellulose
• Digestive enzymes that break down starch
(alpha linkages) can’t break cellulose (beta
linkages) due to shape
• Humans can’t digest! (insoluble fiber)
• What about herbivores (and termites!)??
– Harbor cellulose-digesting prokaryotes in 1st
stomach (rumen)
– Other mammals -
Polysaccharides - Structure
• Chitin Use by arthropods (insects, spiders,
crustaceans) to build exoskeleton
• Also found in fungi cell walls AND dissolving
stitches!
• Similar to cellulose except has
nitrogen-containing group
Polysaccharides - Structure
• Peptidoglycan = another modified
polysaccharide. Used in bacterial cell walls
Lipids!
Lipids
• Class of biomolecules that doesn’t include
true polymers – and not big enough to be
called “macro”
• Mix poorly with water (hydrophobic)
• Mostly consist of nonpolar hydrocarbon
regions (HC)
– Some polar bonds associated with O
• Include waxes, some pigments, fats, and oils
Lipids
• Made up of C, H, and O (notice a pattern??)
• Used for long term energy storage and
insulation
• 3 major groups
– Triglycerides
– Phospholipids
– Steroids
Fats (Triglycerides)
• Triglyceride (or triacylglycerol) is made of one
glycerol & 3 fatty acids.
– Fatty acids = long carbon skeleton
– C—H bonds = reason fatty acids are hydrophobic
• Water molecules bind to each other and exclude the fat
• Connected by dehydration synthesis x 3 (ester
linkages)
Sketch – Simplify
Glycerol
Fatty acid chains
Saturated vs. Unsaturated Fats
• Refers to the bonding of carbon in the fatty
acids.
– Bonding influences shape and properties
• Saturated = no double bonds between
carbons.
– Solid at room temp molecules packed tight
– Food fats (butter, lard)
Saturated vs. Unsaturated Fats
• Unsaturated = at least one double bond.
– Not solid at room temp (oils, plants, fish)
molecules can’t pack tight because of kinks
– Health note – “hydrogenated oil” have been
chemically altered to convert to saturated fats!!
• Which ones are healthier for you?
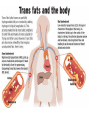
Fats and Health
• Saturated fats contribute to cardiovascular
disease
– Deposits called plaques form on blood vessel
walls, creates bulges and alters flow
• Trans fats Chemically altered unsaturated
but with trans double bonds
– Raise bad cholesterol (LDL) and lower good
cholesterol (HDL)
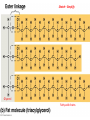
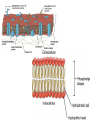
Phospholipids
• Essential structures of cell membranes
• Form fits function (at molecular level)
– Polar heads (face outward, come in contact with
H2O)
– Nonpolar tails (face inward, away
from water)
– When put in water, they
self-assemble into bilayers
– Form semi-permeable membranes,
regulate transport
Steroids
• Lipids with carbon skeleton and 4 fused rings
• Types of steroids vary by chemical groups
attached to rings
• Include hormones and cholesterol
Steroids
• Cholesterol:
– Common in animal cell membranes
– Produced in liver
– Precursor for synthesis of other steroids (including
sex hormones)
– Fats impact cholesterol levels
Proteins!
Proteins
• Foundation of nearly every
dynamic function in an organism
• Account for +50% of dry mass
• Made of C, H, O, N, and some S
• Incredibly diverse and highly
specific
– Humans Tens of thousands,
each with different structure and
function
Proteins
• Most enzymes are proteins Without
enzymes, life could not exists!
– Enzymes = catalysts; speed up chemical reactions
without being used up in the reaction
• “Workhorses” that keep cells running
Proteins Basic Functions
•
•
•
•
•
•
•
•
Enzymes as catalyst for reactions
You’ll create a
Structure/support
Graphic
Organizer for
Storage of amino acids
these!
Transport of substances
Hormones that coordinate activities
Receptors that allow cells to respond to stimuli
Movement via contraction + motor proteins
Protection against disease w/defense proteins
Protein Monomers & Polymers
• Amino Acids building blocks of proteins
– 20 standard AA’s that are part of genetic code
– 21st Selenocysteine (relatively new!)
– Central carbon with 4 attachments
•
•
•
•
Amino group
Carboxyl group
Hydrogen
R-group (varies):
– Also called side chain (important with folding!)
Protein Monomers & Polymers
• Peptide bonds link amino acids together
(dehydration reaction between carboxyl of
one AA and amino group of another)
• Resulting polymer = polypeptide chain
Amino Acids have directionality…
N-Terminus
C-Terminus
Protein Structure
• Diversity of AA’s leads to very diverse 3D
structures of proteins determines function!
• 4 basic levels to structure…
Primary Structure
• Sequence of AA’s in one polypeptide chain
• Determined by genetic code (codon/anticodon
matching of RNA to AA’s)
– Literally “reading” the RNA instructions
• AA’s held together with peptide bonds
Secondary Structure
• Regular, repeating 3D segments of coils or folds
• Form because of hydrogen bonding in the C/N backbone of
the polypeptide chain (thus all proteins have similar
secondary structure)
– All proteins have the same C and N groups
– Result of electronegativity! H’s attracted to O’s
• 2 shapes Alpha helix and Beta pleated sheet
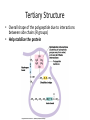
Tertiary Structure
• Overall shape of the polypeptide due to interactions
between side chains (R groups)
• Help stabilize the protein
Tertiary Structure
• Mechanisms:
– Hydrophobic Interaction: AA’s with nonpolar side
chains end up at the core of the structure (away
from water)
• Held together with van der Waals interactions
– H bonds between polar side chains
– Ionic bonds between +/- side chains
– Disulfide Bridges between two cysteine
monomers (those with –SH groups)
• S of one bonds with S of another
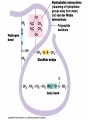
Van der Waals Interactions
• Weak attractions between molecules due to
localized charge fluctuations
• Electrons are not always symmetrically
distributed
– Even nonpolar molecules may have slightly
negative and positive regions
Quaternary Structure
• Two or more polypeptide subunits aggregated
into one functional macromolecule
– Optional level (not all proteins form this)
• Examples:
– Collagen (40% of the proteins in a human body!)
– Hemoglobin
Denaturation
• Change in the structure of a protein
– Changes, reduces, or destroys function
• Conditions that lead to denaturation…
Denaturation
• Change in the structure of a protein
– Changes, reduces, or destroys function
• Conditions that lead to denaturation:
– Temperature (heat)
– pH
– Salt concentration
– Change of overall environment (e.g. aqueous
solution to organic solution)
– Chemicals that disrupt tertiary bonds
The original foundation…
• How might primary structure be altered?
• What happens if primary structure is change?
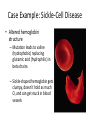
Case Example: Sickle-Cell Disease
• Altered hemoglobin
structure
– Mutation leads to valine
(hydrophobic) replacing
glutamic acid (hydrophilic) in
beta chains
– Sickle-shaped hemoglobin gets
clumpy, doesn’t hold as much
O, and can get stuck in blood
vessels
Understanding Protein Folding
• Incredibly complex – don’t know all patterns or
reasons!
• Several intermediate structures before final
• Methods for tracking involve chaperonin proteins
– Assist in the proper folding of other proteins
Nucleic Acids!
Nucleic Acids
• Blueprint for proteins + information storage
molecules
• Composed of C, H, O, N, and P
• Two types: DNA & RNA
• DNA = genetic material organisms inherit from
parents
Nucleic Acids
• Monomers = nucleotides
• Nucleotide Structure:
– Phosphate + sugar backbone
• Deoxyribose in DNA
• Ribose in RNA
– Nitrogenous base
•
•
•
•
Adenine
Thymine (Uracil in RNA)
Cytosine
Guanine
RNA vs. DNA