* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download water - Lisle CUSD 202
Survey
Document related concepts
Biochemistry wikipedia , lookup
Cell culture wikipedia , lookup
Cellular differentiation wikipedia , lookup
Artificial cell wikipedia , lookup
State switching wikipedia , lookup
Cell growth wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Symbiogenesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell (biology) wikipedia , lookup
Transcript
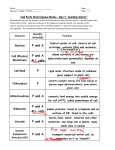
CELLS UNIT Chapter 6: Chemistry in biology Chapter 7: Cellular structure and function Chapter 8: Cellular energy Chapter 9: Cellular Reproduction 6.1 Atoms, Elements, & Compounds Chemistry will help you learn about biology because organisms are made up of different chemicals. Everything is made up of matter and matter is made up of atoms. Atoms are the smallest chemical units of matter. 3 kinds of particles The nucleus is the core part of an atom Proton Positive charge (+) Found in the nucleus Electron Negative charge (-) Travels around the nucleus Neutron Neutral charge Found in the nucleus Elements A scientist named Mendeleev created the Periodic Table which lists all of the known elements on Earth An element is a pure substance made of only one kind of atom Each element is represented by a one or two letter symbol http://www.dayah.com/periodic/ Common elements include carbon, oxygen, hydrogen, helium, nitrogen… Elements differ in the number of protons their atoms contain. Hydrogen only contains one proton Oxygen contains eight protons How many protons does uranium have? Atomic Number and Atomic Mass Atomic number tells us how many protons are in an element. Atomic mass tells us how many protons and neutrons are in the nucleus (how heavy the element is) Compounds A stable substance made up of two or more elements. Every compound is represented by a chemical formula. Each element may have a subscript that tells how many there is of that element. Water H2O H= white O= red How to count #’s of elements in compounds Examples: salt (NaCl, 1 sodium and 1 chlorine) water (H2O, 2 hydrogen and __ oxygen) carbon dioxide (CO2, __ carbon and __ oxygen) sugar (C6H12O6, __ carbon, __ hydrogen __ oxygen) Bonding (2 types) Covalent bond – when two or more atoms share electrons and form a molecule. A molecule is a group of atoms put together. The number of protons found in the nucleus should equal the number of electrons floating around the nucleus. This makes an element stable. Ionic Bond – A bond formed between molecules when electrons are transferred. Sometimes atoms or molecules gain or lose electrons This transfer causes one atom to be positive and one atom to be negative. When combined they form a stable molecule (like salt, NaCl). 6.2 Chemical Reactions Chemical reactions allow living things to grow, develop, reproduce, and adapt. Chemical reaction – process by which atoms or groups of atoms in substances are reorganized into different substances. Energy is the ability to move or change matter. Energy can be stored or released by chemical reactions. Chemical Equations The starting materials for a chemical reaction are called reactants. The newly formed substances created after a reaction are the products. REACTANTS C6H12O6 + O2 PRODUCTS CO2 + H20 glucose and oxygen react to form carbon dioxide and water Circle the reactant(s) and box the product(s) H2O + NaCl (salt) ----------> Na+ + Cl- H2CO3 (carbonic acid) <------- H2O + CO2 HCl + NaOH -----------------> NaCl + H2O Balanced equations In chemical reactions, matter cannot be created or destroyed. This means that the number of atoms of each element on the reactant side must equal the number of atoms of the same element on the product side. Balance this equation: C6H12O6 + O2 CO2 + H20 Energy of Reactions Living things cannot undergo chemical reactions without energy. Activation energy – the maximum amount of energy needed for reactants to form products in a chemical reaction. Enzymes Enzymes are proteins that speed up chemical reactions in the body by lowering the activation energy. Enzymes are never used up in the reaction. They can be used again and again. Lock-and-Key Model of Enzymes The reactants that bind to the enzyme are called substrates. The specific location where a substrate binds on an enzyme is called the active site. Factors that Affect Enzyme Function Enzymes operate best within certain temperature ranges. Temperatures outside this range make the reaction move slowly or not at all. Enzymes operate best within a certain range of pH values. Too low of a pH (acidic) or too high of a pH (basic) will slow or stop a reaction. 6.3 Acids and Bases Acids – any substance that releases hydrogen ions (H+) when dissolved in water. Bases – any substance that releases hydroxide ions (OH-) when dissolved in water. The amount of hydrogen or hydroxide ions determines the strength or weakness of the substance. The pH scale is used to determine if a substance is an acid or base. The pH scale ranges from 0 to 14. Acids have values from 0 – 6. Bases have values from 8 – 14. Anything with a value of 7 is considered neutral. 6.4 Carbon Compounds Carbon compounds are also known as organic compounds. They make up most of what living things are made of. These compounds are made up of many carbon molecules covalently bonded to each other and to other elements (usually hydrogen & oxygen) Carbohydrates also known as sugars made up of carbon, hydrogen and oxygen molecules in a ratio of 1:2:1 glucose is a common biological sugar (C6H12O6) examples: sucrose, lactose, glycogen, cellulose functions: provide energy, structural support Lipids include fats and oils they do not dissolve in water made up of lots of carbon and hydrogen connected in long chains examples: phospholipids, steroids functions: store energy, provide support, insulation Some lipids contribute to cardiovascular disease by lining the blood vessels with plaque. Plaque build up can block blood flow causing a stroke or heart attack. Proteins made up of smaller molecules called amino acids enzymes are a type of protein used to speed up chemical reactions. salivase is an enzyme in the mouth that helps to break down food that you chew lactase is an enzyme in the stomach that helps to break down lactose found in dairy products functions: transport substances, speed reactions, structural support, control cell growth Nucleic Acids made up of smaller molecules called nucleotides nucleotide = sugar + phosphate + base examples: DNA, RNA function: store and transmit genetic information 7.1 Cell Discovery and Theory Robert Hooke discovered cells using a simple microscope in 1665. He called them cells because they reminded him of small rooms where monks live. Cell Theory In 1838, Matthias Schleiden and Theodor Schwann concluded all living things are made of one or more cells. In 1855, Rudolph Virchow proposed all cells come from other living cells. Many years later, scientists concluded that cells give living organisms structure. Characteristics of Microscopes Since cells are not able to be seen by our eyes, we need microscopes to magnify them. Microscopes enable biologists to examine the details of cell structure and to understand how organisms function. Types of Microscopes Compound Light Microscopes have a low magnification and can be used to examine living cells. Electron Microscopes have a high magnification but cannot be used to examine living cells. Scanning Tunneling Microscopes use a computer to generate a three-dimensional image of the object. Basic Cell Types Prokaryotic cells are the smallest and simplest cells on Earth. The best example of a prokaryote is bacteria. Characteristics of Prokaryotes surrounded by a cell wall DNA moving freely inside the cell some have flagella to help them move don’t have a nucleus Eukaryotic cells are larger and more complex. They have a nucleus and other organelles. Cell Animation 7.2 Cell Membrane The cell membrane helps to maintain a cell’s homeostasis. The cell membrane is selectively permeable meaning it allows only certain substances into and out of the cell. The cell membrane is made up of a double fat layer called a phospholipid bilayer. Draw a diagram of a cell membrane in your notes. Membrane Proteins Proteins in cell membranes include: enzymes which help with chemical reactions inside the cell receptor proteins which pull substances into the cell when the cell needs it transport proteins which help move substances across the membrane either into or out of the cell 7.3 Organelles cytoskeleton: eukaryotic cells have a cytoskeleton of microscopic protein fibers that provide structure and support for the cell and its organelles nucleus: tells the cell what to do and stores DNA the nucleus is surrounded by a double membrane called the nuclear envelope that helps protect the DNA inside when a cell prepares to divide, the DNA inside the nucleus forms chromosomes. This helps genetic information get transferred from the old cell to the new cell. Production of Proteins proteins are made or created inside ribosomes which are found on another cell organelle called the endoplasmic reticulum (ER) proteins are important because they help with chemical reactions Distribution of Proteins proteins must be programmed so they know what job to perform inside the cell the Golgi apparatus is the organelle that programs proteins Lysosomes some proteins are special because they help breakdown and digest substances inside the cell Mitochondria make and store energy from carbon compounds (carbohydrates, lipids, proteins) The energy stored is ATP Which types of body cells would need the most mitochondria to make energy for the body? Structures in Plant Cells Cell Wall used for support and protection maintains an upright shape for all plants Chloroplasts structures that give plants their green color help to capture energy from the Sun to make food Central vacuole used to store excess water for the plant when the environment gets dry also helps give a plant it’s shape 7.4 Cellular Transport Cells have to maintain a stable internal environment in order to survive. We call this homeostasis. Cells are constantly bombarded by their external environment. The cell membrane’s job is to control what goes in and what comes out of the cell. Passive Transport Passive transport - when something passes through the cell membrane without using any energy Equilibrium – state of balance when a substance on one side of the cell membrane equals the amount on the other side Concentration gradient – when one side of the membrane has a higher concentration of substances than the other side Diffusion Particles or substances inside and around the cell constantly move. Diffusion – when substances move from an area of high concentration to an area of low concentration. The cell does not have to use any energy for a substance to diffuse into or out of a cell. Remember that the cell membrane is selectively permeable; that means it only allows certain substances to pass through. Facilitated Diffusion Most cells have membrane proteins embedded in their cell membrane that help to bring in or carry out substances. A carrier protein is a molecule that typically carries amino acids and sugars across the cell membrane. (too big to move on their own). Another word for facilitate is “to help”. The cell does not have to use any energy for a substance to diffuse into or out of a cell. Osmosis Osmosis is the diffusion of water across a selectively permeable membrane. Water is always needed by the cell, so it passes easily through the cell membrane. A cell always wants to be stable, so water will move into and out of a cell until the inside of the cell is neutral (pH of 7) How does water move? There are three possibilities for the direction of water movement across a cell membrane: Water moves out Water moves in Water does not move Isotonic Solutions When water is equal on either side of the cell membrane, there is NO MOVEMENT of water into or out of the cell. The cell shape remains unchanged. This is the condition most cells try to maintain in order to survive. Hypotonic Solutions When the concentration of water outside of the cell is greater than inside the cell, the water MOVES INTO the cell. When water moves in, the cell swells and sometimes bursts. Because plant cells are more rigid than animal cells, they typically don’t burst. Hypertonic Solutions When the concentration of water outside of the cell is less than inside the cell, the water MOVES OUT of the cell. When water moves out, the cell shrinks. Active Transport Active transport requires the cell to use energy to move substances against a concentration gradient. The energy the cell uses is ATP. Substances have to move from an area of low concentration to an area of high concentration. Swimming pool, going up a down escalator, kayaking demo... Types of Active Transport Proteins and large sugars are too large to pass through the membrane or be moved by membrane proteins. These substances are moved across a cell membrane by vesicles (large pockets in the cell membrane). Draw diagrams in your notes active transport demo 8.1 Obtaining Energy In order for your body to function properly, you need energy to perform daily activities. All energy comes from the Sun. Autotrophs make their own food by using the Sun’s energy Heterotrophs need to ingest food to obtain energy Metabolism Metabolism – all the chemical reactions that take place in a cell Two important chemical reactions involve: photosynthesis cellular respiration ATP Adenosine triphosphate is the most important biological molecule that provides energy for living things. ATP releases energy when the bond between phosphates is broken. 8.2 Photosynthesis Photosynthesis – describes the process of how the Sun’s energy is trapped and converted into energy Overall equation: SUNLIGHT 6CO2 + 6H2O C6H12O6 + 6O2 Plants have special organelles called chloroplasts that help to capture the Sun’s energy. 8.3 Cellular Respiration Cellular respiration – describes the process of how living organisms obtain energy by breaking down organic molecules Overall equation: C6H12O6 + 6O2 6CO2 + 6H2O + energy Animals have special organelles called mitochondria that help to breakdown sugars into useable energy. carbon dioxide and water glucose and oxygen 9.1 Cellular Growth Cell size is limited by its ratio of surface area to its volume. Surface area – the area covered by the cell membrane. Volume – space taken by the inner contents of the cell. Importance of Small Cell Size As a cell grows, its volume increases much more rapidly than the surface area. By remaining small, cells can function better. Substances can move in and out easily The cell can communicate across smaller distances The Cell Cycle Once a cell reaches its size limit, something must happen—either it will stop growing or it will divide. Most cells will eventually divide. Cell cycle – a repeating sequence of events that allow a cell to grow and divide. How do cells know when to divide? Just as traffic lights control the flow of traffic, cells have a system that controls the phases of the cell cycle. Cells have a number of “red light-green light” switches that regulate information traveling through the cell. Cells can’t divide unless they pass all checkpoints with green lights. Yellow or red lights would slow or stop cell division. Stages of the Cell Cycle Chromosomes How many cells do you think are produced by the human body everyday? 2 trillion cells, that’s 25 million cells every second Why do cells divide? Cells need to grow, develop and repair themselves When a cell divides, the DNA must be copied before the genetic information is distributed Prokaryotic Cell Reproduction Bacteria have a single circular strand of DNA that “floats” around the cell; the DNA is not contained within a nucleus Prokaryotes reproduce by a type of cell division called binary fission. Binary Fission Eukaryotic Cell Reproduction In eukaryotes, DNA is organized into units called genes Genes are small segments of DNA A single molecule of DNA has thousands of genes lined up next to each other Chromosomes When a cell prepares to divide, the DNA coils up into a structure called a chromosome. Each chromosome has two strands; each strand is an exact copy of the other. Each individual strand is called a chromatid. The two chromatids are connected by a point called a centromere. Chromosome Structure Homologous chromosomes are those that are identical in structure. Most humans have 23 pairs of chromosomes (46 total). There are two types of cells: somatic (body cells) and gametes (sex cells). Cancer Sometimes cells have mutated chromosomes that lead to cancer. These cancer cells can change all the checkpoints to green lights and they coast through the cell cycle reproducing rapidly. All cancers are different, but if scientists can figure out what changes all the checkpoints to green lights we could cure cancer. Causes of Cancer Carcinogens – substances that are known to cause cancer. Asbestos Tobacco products Ultraviolet radiation and other forms of radiation Genetics