* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download radiation physics
Plasma (physics) wikipedia , lookup
Electron mobility wikipedia , lookup
Length contraction wikipedia , lookup
Fundamental interaction wikipedia , lookup
Lorentz force wikipedia , lookup
Electromagnetism wikipedia , lookup
Nuclear physics wikipedia , lookup
Weightlessness wikipedia , lookup
State of matter wikipedia , lookup
Atomic nucleus wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Atomic theory wikipedia , lookup
Metallic bonding wikipedia , lookup
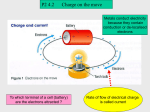
Rad 160 - Radiographic Physics Unit 2 - Electrostatics I. Electrostatics – The branch of physics that deals with stationary or fixed electric charges. Also called Static Electricity. A. Two Types of Electric Charge 1. Positive Charge – atoms lose an electron A positively charged object has a deficiency of electrons 2. Negative Charge – atoms gain an electron A negatively charged object has an excess of electrons *A charged body has an electric field surrounding it. II. Electrification – a process where electrons are removed or added to a body of matter. (Electrons are transferred.) A. Matter becomes positively or negatively charged. B. Matter is “electrified” (or charged). C. Electrification is similar to ionization but with electrification is a whole body and ionization is individual atoms. D. Ultimately, matter is electrically neutral because in the entire universe the total number of negative charges equals the total number of positive charges. E. Why do only electrons move? Why not transfer protons? Because electrons travel in shells around the nucleus of atoms and can be moved. Protons are in the nucleus of atoms and cannot be removed without specialized equipment. III. Five Laws of Electrostatics A. How electric charges interact with each other. 1. Like charges repel; unlike charges attract. The electric field surrounding a charged body radiates outward from a positive charge and inward towards a negative charge. (Bushong, p. 64 Figure 4-6) 2. The electrostatic “force” between two charges is directly proportional to the product of their quantities (charges) and inversely proportional to the square of the distance between them. Force – may be attraction of repulsion depending on whether the charges are different or alike. Example 1: The stronger the charges, the stronger their electrical attraction of repelling force. Example 2: If the distance between the two charges increases, their force of attraction or repulsion decreases. 3. Electric charges reside only on external surfaces of conductors because the repulsion of like charges, the charges move to the outer surface of the object which provides the greatest distance between them. (Selmans, p. 42) Example: A negatively charged metallic ball The negative charges move as far away from each other as possible. *Charges move to outer surface in conductors only. In non-conductors there is equal distribution throughout. 4. The concentration of charges on a curved surface of a conductor is greatest where curvature is greatest. (Selmans, p. 43) Example: There are more charges concentrated on the sharpest curvature. 5. Only negative charges (electrons) can move in solid conductors. IV. Grounding - - creating a pathway for electrons to move between a charged body and the earth. Neutralizing a charged body by grounding it or connected to wet earth by a conductor. (Selmans, p. 41) The earth – has an infinite number of charges within it. A. What happens when you ground an object? 1. The object and the earth must be connected with a conductor (Ex: a metallic wire) that allows easy movement of electrons. 2. If the object is negatively charged, electrons move down the conductor into the earth making the body neutral. 3. If the object is positively charged, electrons move up the conductor from the earth to the charged body making it neutral. Basically, the charged body is neutralized by being brought to the same electrical potential energy level as the ground. V. Methods of Electrification A. Methods of removing or adding electrons 1. Friction - The removal of electrons from one object by rubbing it with another of a different kind. Simplest method of electrification. Example: When you walk on carpet electrons are removed from the carpet to your shoes. You are negatively electrified. Object is electrified if it has too many or too few electrons. 2. Contact - When a charged body touches an uncharged body, the uncharged object acquires the same charge. Example: In the previous friction example: You are negatively “charged by friction” from walking on carpet. You touch a doorknob and get shocked as you transfer these excess electrons to knob. The knob is now “negatively charged by contact.” 3. Induction - The electric field surrounding a charged body (the body can be either positively or negatively charged) can electrify an uncharged metallic object with a charge that is opposite of that of the originally charged body. VI. The Process of Electrification by Induction (Selmans, p. 40) A. If a body of matter is charged, it will have an electric field surrounding it. (Think of the invisible force fields the crew members of Star Trek use to use to protect themselves from evil aliens.) B. When an uncharged metallic object is brought into the electric field of a charged object, it experiences a shift of electrons within the object. Note: there is no contact between the 2 objects. C. Depending on the charge of the charged object, the shifting electrons will move towards or away from the charged object (only the electrons move). Remember: Opposites attract, likes repel. Example 1: If the charged object is positively charged: Example 2: If the charged object is negatively charged: D. A metallic object with all of its electrons (negative charges) shifted to one side. E. Ground the end of the object farthest away from the originally charged object, electrons will shift, depending upon the original charges. F. Remove the ground and the metallic object will be charged. In the above example, the metallic object will be positively charged. VII. Electrical Conductivity of Matter – How readily it allows the flow of electrons. A. Three Classifications of Conductivity – (Bushong, p. 67 Table 4-1) 1. Non-Conductors – (insulators) electrons do not flow freely in them. Example: Rubber, plastic, glass 2. Semi-Conductors – allows an average flow of electrons Example: Silicon 3. Conductors – electrons flow freely Example: Metals – silver, copper, aluminum B. Electric Field – The zone surrounding a charged body. Responsible for electrification by induction of an uncharged object. C. Electroscope – A device that detects the presence of electric charge. *Experiment with scope (Selmans, p. 43) D. Static Discharge – The transfer of electrons between bodies of opposite charges. 1. The two want to come together but there is a space (insulator) between them. 2. The opposite electric charges continue to build up. 3. Eventually the build-up is great enough that the negatively charged electrons jump to the less negative body. Eventually, continued buildup of electric charge causes breakdown of the insulator as the electrons jump from the more negative to the less negative body, producing a spark. Example: Lightening Objectives 1. Define electrostatics. 2. Explain electrification and the methods of electrification. 3. List the classifications of conductivity and an example of each. 4. Define electric field. 5. List and explain the five laws of electrostatics. 6. Explain static discharge. Reading Selmans – Chapter 5 & Bushong pgs 61-65. This workforce solution was funded by a grant awarded by the U.S. Department of Labor's Employment and Training Administration. The solution was created by the grantee and does not necessarily reflect the official position of the U.S. Department of Labor. The Department of Labor makes no guarantees, warranties, or assurances of any kind, express or implied, with respect to such information, including any information on linked sites and including, but not limited to, accuracy of the information or its completeness, timeliness, usefulness, adequacy, continued availability, or ownership.