* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ratio of Charge to Mass (e/m) for the Electron
Magnetic monopole wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Electromagnetism wikipedia , lookup
Condensed matter physics wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Superconductivity wikipedia , lookup
Lorentz force wikipedia , lookup
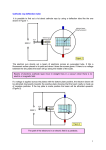
Ph 122 quark%/~bland/docs/manuals/ph122/eoverm/eoverm.doc June 26, 2017 Ratio of Charge to Mass (e/m) for the Electron In this experiment we observe the motion of free electrons in a vacuum tube. From their response to electric and magnetic fields the ratio of charge to mass for the electron can be determined. At the turn of the century several crucial experiments were performed to demonstrate the atomic structure of matter. In 1897 J.J. Thompson was able to observe the motion of single electrons in electric and magnetic fields, and so determine the ratio of the electron’s charge, e, to its mass, m. This demonstrated some interesting things about the atom. First of all, this ratio is exactly the same for all electrons, suggesting that they are fundamental particles rather than just fragments of matter. Secondly, since the typical charge on atomic particles was known approximately at the time, the mass of the electron could be estimated, and it turns out to be very tiny compared to the mass of an atom. This was the first suggestion that the electron in an atom might be a small particle orbiting a larger one, like the Earth in the solar system. I. Theory A. The apparatus. This experiment is carried out in a special vacuum tube which contains a small amount of mercury vapor. Electrons emitted by a heated cathode are accelerated by the voltage applied between the cathode and anode. Some of the electrons come out in a narrow beam through a circular hole in the center of the cylinder. This emission is then focused into a narrow beam by the grid of the tube. When electrons of sufficiently high kinetic energy leaving the cathode collide with mercury atoms a fraction of the atoms become ionized. Upon recombination of the ions with stray electrons, a characteristic blue color is observed. This makes the path of the beam of electrons visible as the electrons travel through the mercury vapor. A magnetic field is applied using a pair of coils of wire. Two coils, separated by a distance equal to their radius, are referred to as Helmholz coils. The advantage of this configuration is that it gives a rather uniform field over a rather large area around the centerpoint. The magnetic field at the center of the tube is calculated from a relation given in its manual, B = 7.7 x 10-4 I where I is the current in amps, and B is in Teslas. e/m - 1 B. Electrons in a magnetic field. A charged particle moving in a magnetic field experiences a force which is to the side (perpendicular to the particle’s motion) and perpendicular to the magnetic B (out of field. If the particle’s initial velocity is perpendicular to a paper) uniform magnetic field, it will move in a circle. This situation is shown in figure 1. The magnetic force, equal to evB, is the only force on the electron; so, Newton’s second law (F=ma) gives evB mv 2 r F The direction of the force on the electron is given by the right-hand rule. Walker gives this rule as follows: "To find the direction of the magnetic force on a positive v charge, start by pointing the fingers of your right hand in the direction of the velocity, v. Now, curl your fingers Figure 1. Force on an electron in a toward the direction of B. Your thumb points in the uniform magnetic field. direction of F. If the charge is negative, the force points opposite to the direction or your thumb." You should be able to use this rule to confirm that the force on the electron shown in figure 1 is towards the center of the circle. The velocity v of the electron can be related to the accelerating voltage, using energy conservation: Drop in potential energy gain in kinetic energy eV 1 2 mv 2 Solving this expression for v, and substituting into eB = mv/r, gives us the relationship: e 2V 2 2 (1) m Br II. Experimental Procedure Turn the apparatus on, and wait for warm-up (about one minute). Turn the voltage up to about 200 volts. A beam will now be observed on the glass envelope opposite the anode. Adjust the current through the coils and observe how the beam curves up into the sphere of the tube and eventually bends over to complete a full circle. Measuring the sign of the electron’s charge: First method: Using a compass, determine the direction of the magnetic field applied by the coils. Then, from the direction the electrons bend, determine whether their charge is positive or negative. Explain your reasoning. e/m - 2 Second method: Turn off the current to the coils. Use a permanent magnet with known north and south poles to apply a known magnetic field to the electron beam. From the direction of deflection of the electrons, determine whether their sign is positive or negative. Explain your reasoning. Now turn the magnetic field back on, and bring one end of a bar magnet as close as you can to the electrons' path and observe the spiral path which the electrons now follow. Can you explain why the presence of the extra field distorts the electron's path? How will the Earth's field affect the motion of the electrons? Predict the direction of the bar magnet so as to reduce the radius of the electron's orbit; then try it. r as a function of voltage. Here we plan to measure the radius r of the electron’s circular path as a function of the accelerating voltage V. Note that equation (1) can be solved for V, giving V = B2e/(2m) r2 or y=ax, where y = V, x = r2, and the slope a of the line is given by a = B2e/(2m). Thus if we plot V as a function of r2, the curve should be a straight line, whose slope is related to e/m. Solving for e/m gives e/m = 2a /B2. Set I at 1.5 A. This determines the value of the magnetic field produced by the coils; the relationship, from the equipment manual, is B = k I, where k = 7.7 x 10-4 T/A . Calculate the value of B corresponding to the current you have set. Then vary the accelerating voltage V and measure r, the radius of the orbit, as a function of V, over the widest range of voltages for which a measurement is feasible. The most convenient way to analyze this data is to open an EXCEL spreadsheet, though plotting by hand is also quite acceptable. See the first experiment, "Data Analysis on the IBM PC," for instructions on plotting and linear fits. Plot V versus r2 and find the slope of the graph (linest is the easiest). Then use your value of the slope to determine e/m, using the relation given above. Note: to get the slope in the right units, r should be expressed in meters. r as a function of current. Here we plan to measure the radius r of the electron’s circular path as a function of the accelerating voltage I, at constant V. Note that if we substitute B = kI into equation (1), it can be solved for I, giving I = (2Vm/(k2e))1/2 (1/r) Thus if we plot I as a function of 1/r, the curve should be a straight line, whose slope is related to e/m. In this case the slope a is given by a = (2Vm/(k2e))1/2, and the charge-to-mass ratio is calculated from it using the relation e/m - 3 e/m = 2V/(k2a2). Now set V to a fixed value (200 V). Then determine r for various settings of I, the current through the coils. Plot I against 1/r and find the slope of the graph. Then use your value of the slope to determine e/m, using the relation given above. Note: to get the slope in the right units, r should be expressed in meters. Now you have two experimentally determined values for e/m. Use them to calculate a best value (the average) and the error on the average value. [See the first experiment, "Data Analysis on the IBM PC," for instructions on calculating averages and standard deviations.] State your final result, complete with error. What systematic errors might have been ignored in assigning the error? Calculate the accepted value for e/m. (e = 1.6 x 10-19 C, m = 9.1 x 10-31 kg.) Compare it with your measurement in the standard way (discrepancy, number of sigmas of discrepancy, quality of agreement). Is your result credible? What systematic errors might have been ignored in assigning the error? (What about the Earth's magnetic field?) III. Equipment Daedalon e/m apparatus bar magnet compass e/m - 4





![NAME: Quiz #5: Phys142 1. [4pts] Find the resulting current through](http://s1.studyres.com/store/data/006404813_1-90fcf53f79a7b619eafe061618bfacc1-150x150.png)