* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Do-now
Group 12 element wikipedia , lookup
Alkali metal wikipedia , lookup
Boron group wikipedia , lookup
Alkaline earth metal wikipedia , lookup
Group 3 element wikipedia , lookup
Dmitri Mendeleev wikipedia , lookup
Period 6 element wikipedia , lookup
Period 5 element wikipedia , lookup
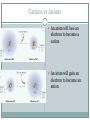
Do-now Get into Lab Groups! Take out a piece of paper! Periodic Table of the Aliens!Figure out what your missing aliens should look like First group to finish gets an EXTRA 5 points! ( Classwork Grade) HINT 1: Look for a pattern on each alien, try to arrange in a grid HINT 2: There should be two ways in which each alien changes HINT 3: Look at the amount of fingers on each alien! Group similar ones together and look for another pattern Chapter 6 THE PERIODIC TABLE Objectives 6.1a Explain how chemists began to organize the known elements. 6.1b Describe how Mendeleev organized his periodic table. 6.1c Describe how the modern periodic table is organized. 6.1d Identify three broad classes of elements. QUESTION What makes one element different from another? The number of protons in the nucleus! Organizing the elements Early Chemists, such as JW Dobereiner, used properties of elements to sort them into groups “triads” were sets of three elements with the same properties For instance- Chlorine, Bromine, and Iodine Mendeleev Dmitri Mendeleev was a Russian chemistry teacher who published his own table of the elements In his table he arranged each element by increasing atomic mass and noticed patterns There were unknown elements in his table that had not been discovered yet! The modern periodic table- p. 162 Unlike Mendeleev’s table, the modern periodic table is not measured by increasing atomic mass Instead, atomic number is used We see a new pattern, the periodic law, where as the elements are listed in increasing atomic number, there properties will repeat periodically. Atomic number increases down and to the right A closer look Three classes of elements Metals 2. Nonmetals 3. Metalloids 1. Metals Metals- good conductors of heat and electric current High luster or “sheen” Solid at room temperature, except for mercury Ductile and malleable How it’s made- Aluminum Nonmetals Nonmetals- poor conductors of heat and electric current Carbon is an exception to this rule Typically gases at room temperature Solid nonmetals are typically brittle Periodic Videos- Chlorine https://www.youtube.com/watch?v=BXCf Bl4rmh0 Metalloid Metalloid- has properties similar to those of metals and nonmetals Under some conditions behave as a metal, under others, behave as a nonmetal How it’s made- computer chips https://www.youtube.com/watch?v=aWVywhzuHnQ Homework Worksheet 6.1 Do-now Hand in Homework! Write the electron configurations for Helium, Neon, Argon, and Krypton in your notes. Objectives 6.2a Describe the information in a periodic table. 6.2b Classify elements based on electron configuration. Relating to electron configuration Write the electron configuration for Helium, Neon, Argon, and Krypton Helium Neon Argon Krypton What do each of these have in common? Groups of Elements Groups of Elements Alkali Metals (1A) Alkaline Earth Metals (2A) Halogens (7A) Noble Gases (8A) Alkali Metals Noble Gases Blocks of Elements “s” block1A-2A “p” block- 3A-8A “d” block- all B groups “f” block Electron Configuration Groups All noble gases have completely filled electron configurations Elements in groups 1A through 7A (s and p blocks) are usually referred to as representative elements Sometimes called “main group elements” The B group elements (d block) are called transition metals. The lower block elements (f block) are called inner transition metals. Blocks of Elements “s” block1A-2A “p” block- 3A-8A “d” block- all B groups “f” block Electron Configuration Groups Representative Elements Transition Metals Inner Transition Metals Noble Gases Repre sentat ive NOBLE GAS CONFIGURATION Selenium Barium Iron Copper Electron configuration in groups WHERE IS ELEMENT: 1s2, 2s2, 2p6, 3s2, 3p6, 4s1? [Xe] 6s2, 4f5 ? Battleship! Rules: Set up your fleet. Player 1 calls out an element by stating its noble gas configuration Player 2 confirms this by stating “hit” or “miss” If a hit, player 1 gets another shot! Play until someone’s whole fleet has been sunk! Have fun! Homework Read section 6.2 Lesson Check, p. 171 Quiz Tomorrow Lab Tomorrow Projects due tomorrow Activity- Periodic Trends Download a periodic table app on your phone Your will be given a property for that element Atomic radius (size) Ionization energy or First Ionization energy Ionic radius (size) Electronegativity Find the values for elements 1-36 (H-Kr), arrange in a table form. Graph those elements as property vs. atomic number. (2 graphs!) Put a title on the graph and label the axes Objectives 6.3a Describe trends among elements for atomic size 6.3b Explain how ions form 6.3c Describe periodic trends for first ionization energy, ionic size, and electronegativity Trends in the Periodic Table The periodic table is organized by atomic number and by electron configuration Other patterns begin to occur! Ionization Energy Atomic Radius Electronegativity Atomic Radius Atomic Radius tends to increase from top to bottom in a group and decreases from left to right in a period Ions Ions are atoms that have either a positive or negative charge They have an unequal amount of protons and electrons Positive and negative ions form when electrons are transferred in between atoms Cations: Ions with a positive charge Anions: Ions with a negative charge Sodium and Chlorine HTTPS://WWW.YOUTUBE.COM/WATCH?V=MX 5JJWI2AAW Cations vs Anions An atom will lose an electron to become a cation An atom will gain an electron to become an anion Demo I NEED 3 POSITIVE STUDENTS AND 3 NEGATIVE STUDENS Ionization Energy The ionization energy is the energy required to remove the first electron from an atom Table on p. 177 Will decrease from top to bottom in a group and increase from left to right across a period Demos Sodium in Water Potassium in Water Rubidium in Water Cesium in Water Cesium in Water (slow-mo) Ionic Size The size of ions tends to increase from top to bottom within a group. They will decrease from left to right across a period Electronegativity Electronegativity is the ability of an atom of an element to attract electrons when the element is in a compound Defined by Linus Pauling Electronegativity values tend to decrease from top to bottom in a group, and increase from left to right in a period Review How does ionization energy increase? What will produce larger ions, Oxygen or Nitrogen? What has a larger atomic radius, Cesium or Potassium? Which is more likely to attract more electrons, Silicon or Sulfur? Homework Finish Graph- to be handed in