* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Superfluid Helium
Relativistic quantum mechanics wikipedia , lookup
Identical particles wikipedia , lookup
Quantum state wikipedia , lookup
Elementary particle wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Hidden variable theory wikipedia , lookup
Canonical quantization wikipedia , lookup
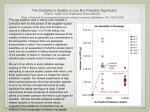
Volume 24, Number 13 PHYSICAL REVIEW LETTERS 20 November 2003 Superfluid Helium Gray O’Byrne Department of Physics, University of Ottawa, Ottawa Ontario, K1S 5M2 Canada (Received 27 April 2003) When one lowers the temperature of most substances the attractive forces between atoms eventually prevail over the repulsive forces and a solid is formed. In helium however, these attractive forces are too weak for this to occur. At 4.2K helium will become a liquid, but at atmospheric pressure it will never solidify. This allows us to cool helium in the liquid phase to temperatures much lower than other substances. At these low temperatures liquid helium develops some interesting and unique properties such as the lack of viscosity and near perfect thermal conductivity. Kapitza, one of the leading researchers in the field coined the word "superfluidity" to describe this behavior. The uniqueness of helium to demonstrate these properties, as well as the quantum effects allowing them will be introduced. Also, differences and similarities between the isotopes of helium as well as some history of superfluidity will be discussed.[S0031-9O07(03)02379-X] PACS numbers: 82.25.+z, 05.40.+j.83.10.Nn, 87.15.He In 1908 Heike Kamerlingh Onnes reduced the temperature of helium below 4.2K and so, for the first time, was able to liquefy it.[1] (For this work, he received the Nobel Prize in 1913) Onnes continued to lower the temperature of helium hoping that it would eventually solidify. His efforts however, were doomed to failure. The fact is that helium will never solidify at atmospheric pressure. The cause of this is Quantum Mechanics and the Uncertainty Principle.[2] As one lowers the temperature of a substance its atoms slow down and in a classical model they will reach a full stop at absolute zero. In other words, the substance will reach a state of zero kinetic energy at absolute zero. This however, is not the full picture. The uncertainty principle tells us that we cannot have stopped particles neatly placed in a substance. Even at absolute zero all particles must have a small, minimal uncertainty on their momentum. This momentum is equivalent to these particles moving around a little. This means they have a small amount of kinetic energy, even at absolute zero. We call this energy the zero-point energy. Helium is the only substance in which the zero-point energy has a considerable effect. The combination of the small mass of helium and the week Van der Waals forces between atoms allow helium to remain in the liquid phase even at its zeropoint energy. Helium actually can be solidified, as seen in Figure.1, but it is only at high pressures (about 25 atmospheres) that its atoms are finally pushed close enough together to do so. I would like to note that there are actually two stable isotopes of helium. It was 4He that was used by Onnes and by others in early experiments with liquid helium. 3He, as we will see later, has different properties. 1170 0021-9007/02/78/63/1170(4)$10.00 FIG. 1. Phase Diagram of 4He. One can note the absence of a triple point which leads to the liquid phase continuing all the way down to absolute zero. Despite Kamerlingh Onnes reducing the temperature of liquid helium to below 1k, it was not until 1937 that a second phase transition was observed. This unusual occurrence was first recorded by Peter Kapitza who noticed, among other things, that helium below 2.17K could easily flow through the smallest pores, even ones too small to permit regular helium. Kapitza coined the word “superfluid”[3] to describe this new state. He received one half of the Nobel Prize in 1978, in part due to this discovery. Many other discoveries were made concerning liquid helium in following years. One of these was the behaviour of the specific heat of the liquid (Figure.2.) We can clearly see that the specific heat has an asymptote at 2.17K. This temperature is called the lambda point due to the resemblance of the graph to the Greek letter lambda (.) © 2003 The American Physical Society 1170 Volume 24, Number 13 PHYSICAL REVIEW LETTERS The lambda point marks the ‘second order’ phase transition in liquid helium. The properties of the fluids on either side of the lambda point are so different that the liquid above 2.17K is referred to as He I, and the fluid below 2.17K is referred to as He II. FIG. 2. Lambda Point in 4He. This marks the phase transition to He II. He II has many unique properties. In their early experiments J.F.Allen and A.D.Misener [4] measured an upper limit of the viscosity of He II at 1.07K to be = 4*10-9. Compared to hydrogen gas (previously thought to be the least viscous substance) this is around 104 times less viscous. J.F.Allen accidentally made another important discovery by shinning a flashlight on his apparatus. When illuminated by his flashlight the helium shot up and out of his apparatus. This is now known as the fountain effect.[5] Perhaps the most easily viewed property of He II is its near perfect thermal conductivity. This is because helium simply stops boiling when brought below the lambda point. Boiling normally occurs because localised parts of a liquid get heated enough to vaporise. These pockets do not form in He II because any added heat is instantly and evenly distributed throughout the liquid. Instead we have smooth uniform evaporation from the surface. I would at last like to mention two, somewhat more bizarre properties of superfluid helium. The first is that it cannot be kept in an open vessel because it will simply creep up the sides as a film and climb out. Left alone, an open vessel of He II will actually empty itself. The second is that when we rotate a container of He II the liquid does not spin with it. Instead a pattern of whirlpools called vortices are formed.[6] These vortices are very small. Their diameters measure only 1000 Å to 100,000 Å. Table.1 The Two-Fluid Model Normal fluid Superfluid Density N s (< N) Viscosity N N = 0 Entropy SN SN = 0 1170 0021-9007/02/78/63/1170(4)$10.00 20 November 2003 To better understand He II, I will introduce the two fluids model. In this model He II is a homogeneous mixture of a superfluid and a normal fluid. The properties of each are summed up in Table 1. At the lambda point He II is 100% normal fluid, but at 1K it is already almost entirely superfluid as shown in Figure 3. We cannot, however, think of this as a mixture in the normal sense. For example we cannot separate the two fluids. We cannot even really say which part is superfluid and which normal. He II simply has properties of both. Heating He II has the expected (and boring) effect of converting superfluid to liquid helium, but this leads to some interesting phenomena. As mentioned above He II is homogeneous, and so, when locally heated, superfluid helium from the non heated sections will immediately flow to the heat source, effectively causing cooling. This is why He II has such a good thermal conductivity (several hundred times that of pure copper!) [7] This also explains the fountain effect, when Jack Allen pointed his flashlight at his experiment he effectively heated a section of it and caused liquid to rush to the lighted spot. This section soon filled and the only place left for the helium was up and out. Another demonstration of this flow of superfluid is the production of heat waves. By placing a heater and a thermometer at opposite ends of a vessel filled with helium II and applying an AC current to the heater, we can measure heat waves in our vessel as the superfluid and normal fluid rush to either side of the vessel. Fig.3 Densities of the ‘super’ and normal fluids in He II as determined by viscosity measurements. So far we have taken the superfluidity as an experimental fact; but why does 4He at low temperatures possess these bizarre characteristics? The answers to this are rooted in quantum mechanics. The Third Law of Thermodynamics in one form states that the entropy of any substance goes to zero as its temperature goes to zero. The following statistical interpretation of entropy was formulated by Boltzmann S k B ln( ) © 2003 The American Physical Society 1170 Volume 24, Number 13 PHYSICAL REVIEW LETTERS where S is the entropy and Ω is the number of quantum states that the system can achieve. This implies that any zero entropy system has Ω=1. In other words any system must collapse to a unique ground state as temperature goes to absolute zero. But what is this state? For simplicity we will start by considering only the case of 4He. 4He is what is known as a boson. In brief bosons are all particles with integer spin. Bosons do not obey the Pauli Exclusion Principle, and so, they can share a common quantum state. This is exactly what happens when we cool 4He, the atoms all collapse to the same ground state. This phenomenon is know as Bose-Einstein condensation and the collection of atoms in the ground state is called the condensate.[8] In this ‘condensate’ the wavefunctions of the individual particles are all superimposed. We essentially have one great big wavefunction that governs the condensate as a whole. This emergence of a macroscopic wavefunction as the atoms all collapse to the same quantum state is the essence of superfluidity. This means that by disturbing one side of the superfluid you are actually instantly affecting the other side as well, even if they are a macroscopic distance away from one another (a few inches in most experiments!) The condensate acts somewhat like a single giant particle. But unfortunately the macroscopic wave function is not a complete picture of superfluidity either. Interatomic interactions are still significant in He II. However, the unusual properties we have described are manifestations of this quantum effect. 20 November 2003 Superfluidity in 3He was not discovered until the early 1970s when Lee, Osheroff and Richardson were able to cool the liquid to about 2.5mK. These three shared the Nobel Prize in 1996 for this work.[9] The unusual phase diagram of 3He is depicted in Figure 4. One notices not only the absence of a triple point, but also that the solid-liquid line has a minimum around 0.3K. This interesting property is due to the liquid actually being more ordered than the solid below 0.3K. Since the liquid is more ordered, it actually takes energy to solidify it. This means that we can cool 3He by applying pressure (which causes it to solidify.) This method is called “Pomeranchuk cooling” and was the method used by Lee, Osheroff and Richardson in their experiments. But how is superfluidity in 3He even possible? 3 He atoms are fermions and thus have one half spin. This means that they obey the Pauli Exclusion Principle and so, should not be able to undergo Bose-Einstein condensation. The answer as it turns out is rather simple: one half plus one half is one. At very low temperatures the helium particles join together in pairs known as “Cooper Pairs”. The pairing is a result of the polarization of surrounding fluid by a passing atom. A second atom can then come along and sense this disturbance. Weak as this interaction may initially sound, it allows for these pairs to effectively act as bosons which, of course, means they can collapse to a common quantum state. In conclusion, research in the domain of superfluidity continues to be important. The 2003 Nobel Prize in Physics was awarded "for pioneering contributions to the theory of superconductors and superfluids". Superfluids are still helping us develop our picture of the ‘quantum world’. *To whom correspondence should be addressed E-mail [email protected] Figure 4. Phase diagrams of 3He. A-Temperature range from 0 to 3.2K. Notice the minimum in the solid-liquid line around 0.3K. B-Temperature range from 0. to 3mK. The superfluid phase only begins around 2mK. This is a temperature one thousand times lower than required for 4 He. 1170 0021-9007/02/78/63/1170(4)$10.00 [1] Nobel e-Museum (www.noble.se/physics/laurates/1913/onnes-bio.html) [2] Tony Guénault, Basic Superfluids, Taylor & Francis Inc., New York (2003) [3] Pyotr Kapitza, Nature 141, 74 (1938) [4] J.F.Allen & A.D.Misener, Nature 141, 75 (1938) [5] J.F.Allen & H.Jones, Nature 141, 243-244 (1938) [6] Nobel Lectures, 1996 Prize in Physics [7] Kazuyashi Yanaka, Masters Thesis, University of Tsukuba (2003) [8] D.r.Tilley & J.Tilley, Superfluidity and Superconductivity 3rd Ed., IOP Publishing Ltd, London (1990) [9] Gloria B. Lubkin, Physics Today (December 1996) © 2003 The American Physical Society 1170