2 Phase Diagram of 2DES on Liquid Helium
... This work is a contribution to the study of the system of 2-dimensional electrons (2DES) bound to the surface of liquid helium-4. The physical properties of the 2DES are probed through the excitation of normal modes in the radio frequency range 10 MHz-1GHz. The normal mode spectra are expected to un ...
... This work is a contribution to the study of the system of 2-dimensional electrons (2DES) bound to the surface of liquid helium-4. The physical properties of the 2DES are probed through the excitation of normal modes in the radio frequency range 10 MHz-1GHz. The normal mode spectra are expected to un ...
noble gases
... 43. The oxidation state of chromium in the final product formed by the reaction between KI and acidified potassium dichromate solution is a. +4b. +6 c. +2 d. +3 Ans : d K2Cr2O7 is reduced to chromic sulphate, Cr2(SO4)3 in which chromium is in +3 state Vikasana - CET 2012 ...
... 43. The oxidation state of chromium in the final product formed by the reaction between KI and acidified potassium dichromate solution is a. +4b. +6 c. +2 d. +3 Ans : d K2Cr2O7 is reduced to chromic sulphate, Cr2(SO4)3 in which chromium is in +3 state Vikasana - CET 2012 ...
Doubly excited nonautoionizing P, D, and F states of helium with
... B. Results with screened Coulomb interaction ...
... B. Results with screened Coulomb interaction ...
Helium atom - ChaosBook.org
... their mutually attracting or repelling forces? It turns out, we can, but we have to do it with care. The full problem is indeed not accessible in all its detail, but we are able to analyze a somewhat simpler subsystem – collinear helium. This system plays an important role in the classical dynamics ...
... their mutually attracting or repelling forces? It turns out, we can, but we have to do it with care. The full problem is indeed not accessible in all its detail, but we are able to analyze a somewhat simpler subsystem – collinear helium. This system plays an important role in the classical dynamics ...
Helium atom - ChaosBook.org
... their mutually attracting or repelling forces? It turns out, we can, but we have to do it with care. The full problem is indeed not accessible in all its detail, but we are able to analyze a somewhat simpler subsystem – collinear helium. This system plays an important role in the classical dynamics ...
... their mutually attracting or repelling forces? It turns out, we can, but we have to do it with care. The full problem is indeed not accessible in all its detail, but we are able to analyze a somewhat simpler subsystem – collinear helium. This system plays an important role in the classical dynamics ...
Semiclassical theory of helium atom
... quantum chaos theory and in particular the Gutzwiller trace formula. Helium, as the prototype of a two-electron atom, is composed of the nucleus with charge Z=2 and two electrons, see Figure 1. The interplay between the attractive Coulomb interaction between the nucleus and the electrons and the Cou ...
... quantum chaos theory and in particular the Gutzwiller trace formula. Helium, as the prototype of a two-electron atom, is composed of the nucleus with charge Z=2 and two electrons, see Figure 1. The interplay between the attractive Coulomb interaction between the nucleus and the electrons and the Cou ...
helium thermodynamics, analytical model
... are a rewarding topic for the study especially because it is exploited in a number of high-technology applications. On the other hand, helium is one of lightest elements that are ideally used for testing the quantum statistical theories of matter. One of special properties of the most abundant form ...
... are a rewarding topic for the study especially because it is exploited in a number of high-technology applications. On the other hand, helium is one of lightest elements that are ideally used for testing the quantum statistical theories of matter. One of special properties of the most abundant form ...
Symposium Papers - Respiratory Care
... contain 12% of an unidentified gas. In 1907, Cady and McFarland of the University of Kansas published a paper that identified the gas as helium and showed that it could be extracted from natural gas.12 In 1908, Onnes, a Dutch physicist, first liquefied helium, but failed to cool the gas to a solid s ...
... contain 12% of an unidentified gas. In 1907, Cady and McFarland of the University of Kansas published a paper that identified the gas as helium and showed that it could be extracted from natural gas.12 In 1908, Onnes, a Dutch physicist, first liquefied helium, but failed to cool the gas to a solid s ...
INERT GASES -
... The controversy was ended with the subsequent discovery of helium, neon, and the other inert gases. I t was then realized that these gases formed an entirely new group in the periodic table - elements which were characterized by complete chemical inactivity. Apart from radon, which is radioactive, a ...
... The controversy was ended with the subsequent discovery of helium, neon, and the other inert gases. I t was then realized that these gases formed an entirely new group in the periodic table - elements which were characterized by complete chemical inactivity. Apart from radon, which is radioactive, a ...
Existence of an Ericson regime in stretched helium
... mixed phase space @1#. This means that not all of the quantum states of the helium atom can be grouped into regular sequences of states and we should be prepared to discover new quantum dynamical regimes in the helium atom. One consequence of a mixed phase space is the existence of regular islands i ...
... mixed phase space @1#. This means that not all of the quantum states of the helium atom can be grouped into regular sequences of states and we should be prepared to discover new quantum dynamical regimes in the helium atom. One consequence of a mixed phase space is the existence of regular islands i ...
10.4: Helium Atom - PhysWiki
... http://physwiki.ucdavis.edu/Quantum_Mechanics/Fitzpatrick's_Quantum_Mechanics/10%3A_Identical_Particles/10.4%3A_Helium_Atom ...
... http://physwiki.ucdavis.edu/Quantum_Mechanics/Fitzpatrick's_Quantum_Mechanics/10%3A_Identical_Particles/10.4%3A_Helium_Atom ...
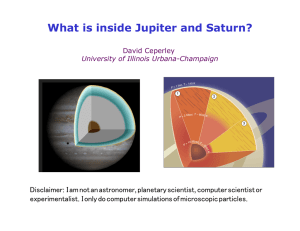
What is inside Jupiter and Saturn? - Physics Illinois
... 1. Is there a liquid-liquid transition in dense hydrogen? 2. How does the atomic/molecular or insulator/ metal transition take place? 3. What are the crystal structures of solid H? 4. Could dense hydrogen be a quantum fluid? What is its melting temperature? 5. Are there superfluid/superconducti ...
... 1. Is there a liquid-liquid transition in dense hydrogen? 2. How does the atomic/molecular or insulator/ metal transition take place? 3. What are the crystal structures of solid H? 4. Could dense hydrogen be a quantum fluid? What is its melting temperature? 5. Are there superfluid/superconducti ...
Superfluid Helium
... a section of it and caused liquid to rush to the lighted spot. This section soon filled and the only place left for the helium was up and out. Another demonstration of this flow of superfluid is the production of heat waves. By placing a heater and a thermometer at opposite ends of a vessel filled w ...
... a section of it and caused liquid to rush to the lighted spot. This section soon filled and the only place left for the helium was up and out. Another demonstration of this flow of superfluid is the production of heat waves. By placing a heater and a thermometer at opposite ends of a vessel filled w ...
HELIUM - IDC
... There are eight known isotopes of helium, but only 3-Helium and 4-Helium Helium are stable. On Earth, 3Helium is present only in traces, mostly since the formation of the Earth. Helium has a valence of zero and is chemically non-reactive under normal conditions and is an electrical insulator unless ...
... There are eight known isotopes of helium, but only 3-Helium and 4-Helium Helium are stable. On Earth, 3Helium is present only in traces, mostly since the formation of the Earth. Helium has a valence of zero and is chemically non-reactive under normal conditions and is an electrical insulator unless ...
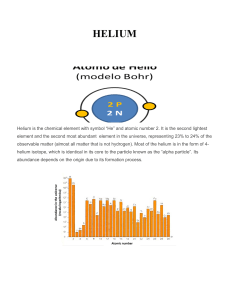
Helium

Helium is a chemical element with symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table. Its boiling and melting points are the lowest among all the elements.Helium is the second lightest element and is the second most abundant element in the observable universe, being present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this figure in the Sun and in Jupiter. This is due to the very high nuclear binding energy (per nucleon) of helium-4 with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. Most helium in the universe is helium-4, and is believed to have been formed during the Big Bang. Large amounts of new helium are being created by nuclear fusion of hydrogen in stars.Helium is named for the Greek god of the Sun, Helios. It was first detected as an unknown yellow spectral line signature in sunlight during a solar eclipse in 1868 by French astronomer Jules Janssen. Janssen is jointly credited with detecting the element along with Norman Lockyer. Jannsen observed during the solar eclipse of 1868 while Lockyer observed from Britain. Lockyer was the first to propose that the line was due to a new element, which he named. The formal discovery of the element was made in 1895 by two Swedish chemists, Per Teodor Cleve and Nils Abraham Langlet, who found helium emanating from the uranium ore cleveite. In 1903, large reserves of helium were found in natural gas fields in parts of the United States, which is by far the largest supplier of the gas today.Liquid helium is used in cryogenics (its largest single use, absorbing about a quarter of production), particularly in the cooling of superconducting magnets, with the main commercial application being in MRI scanners. Helium's other industrial uses—as a pressurizing and purge gas, as a protective atmosphere for arc welding and in processes such as growing crystals to make silicon wafers—account for half of the gas produced. A well-known but minor use is as a lifting gas in balloons and airships. As with any gas whose density differs from that of air, inhaling a small volume of helium temporarily changes the timbre and quality of the human voice. In scientific research, the behavior of the two fluid phases of helium-4 (helium I and helium II) is important to researchers studying quantum mechanics (in particular the property of superfluidity) and to those looking at the phenomena, such as superconductivity, produced in matter near absolute zero.On Earth it is relatively rare — 5.2 ppm by volume in the atmosphere. Most terrestrial helium present today is created by the natural radioactive decay of heavy radioactive elements (thorium and uranium, although there are other examples), as the alpha particles emitted by such decays consist of helium-4 nuclei. This radiogenic helium is trapped with natural gas in concentrations up to 7% by volume, from which it is extracted commercially by a low-temperature separation process called fractional distillation. Helium is a finite resource, and once released into the atmosphere, it readily escapes into space.