* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download NOTES – CHAPTER 4 PYSICAL PROPERTIES – GLASS AND SOIL
History of metamaterials wikipedia , lookup
Condensed matter physics wikipedia , lookup
Acoustic metamaterial wikipedia , lookup
Colloidal crystal wikipedia , lookup
Transparency and translucency wikipedia , lookup
Negative-index metamaterial wikipedia , lookup
State of matter wikipedia , lookup
Glass-to-metal seal wikipedia , lookup
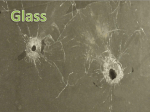
NOTES – CHAPTER 4 PYSICAL PROPERTIES – GLASS AND SOIL HONORS FORENSIC SCIENCE I. Introduction a. Our understanding of the nature of properties can be made easier by classifying them into two categories i. Physical properties = describe a substance without a reference to any other substance 1. Ex. Weight color, boiling point, and melting point ii. Chemical property = describes the behavior of a substance when it reacts or combines with another substance 1. Ex. When wood burns, it chemically combines with oxygen in the air to form new substances 2. Ex. Marquis reagent turns purple in the presence in heroin II. Metric System a. Has basic units of measurement for length (meter) , mass (gram) and volume (liter) b. In the metric system, volume can be defined in terms of length c. At times it may be necessary to convert units from the metric system into the English System or vice versa d. Some of the more useful equivalents: i. 1 inch = 2.54 cm ii. 1 meter = 39.37 inches iii. 1 pound = 453.6 grams iv. 1 liter = 1.06 quarts v. I kilograms = 2.2 pounds III. Physical Properties a. Temperature i. The determination of the physical properties of any material will often require the measurement of temperature ii. Temperature is the measure of heat intensity, or the hotness or coldness of a substance iii. The two most common temperature scales used are the Fahrenheit and Celsius Scales 1. In Fahrenheit, water boils at 212 degrees F and freezes at 32 degrees F 2. In Celsius, water boils at 100 degrees C and freezes at 0 degrees C b. Weight and Mass i. The force with which gravity attracts a body is called weight ii. Mass differs from weight because it refers to the amount of matter an object contains and is independent to its location on earth or any other place in the universe iii. In the metric system, the basic unit of mass is the gram iv. The mass of an object is determined by comparing it against the know mass of standard objects v. The comparison is performedon a balance c. Density i. Is defined as mass per unit volume ii. Is an intensive property of matter – is the same regardless of the size of a substance and is a characteristic property of a substance and can be used as an aid in identification iii. Solids are more dense than liquids, and liquids more dense than gases iv. The volumes of gases and liquids vary considerably with temperature so when determining density, it is important to control and record the temperature at which the measurements are made d. Refractive Index i. Light waves travel in air at a constant velocity of nearly 300 million meters per second until they penetrate another medium ii. When light waves hit another medium, they slow, causing the rays to bend iii. The bending of a light wave because of a change in velocity is call refraction iv. The ratio of the velocity of light in a vacuum to that in any medium determines the refractive index v. Like density, the refractive index is an intensive physical property of matter and can serve to characterize a substance vi. Tests used to determine a substance’s refractive index must be performed under carefully controlled temperature and lighting conditions vii. Normally a solid or a liquid would be expected to exhibit only one refractive index value however, many solids that are crystalline in nature will have two refractive indices whose values in part depend on the direction in which the light enters the crystal with respect to the crystal axis. IV. V. 1. Crystalline solids – have definite geometric forms because of the orderly arrangement of the fundamental particle of a solid, the atom viii. Not all solids are crystalline in nature, some are amorphous 1. Amorphous solids – a solid win which the constituent atoms or molecules are arranged in random or disordered patterns 2. Glass is an amorphous solid ix. Other characteristics of glass 1. Birefringence – a difference in the two indices of refraction exhibited by most crystalline materials 2. Dispersion – the separation of light into its component wavelengths Comparing Glass Fragments a. Glass that is broken and shattered into fragments and minute particles during the commission of a crime can be used to place a suspect at the crime scene b. Glass is a hard, brittle, amorphous substance that is composed of silicon oxides mixed with various metal oxides. c. Tempered glass = glass to which strength is added by introducing stress through the rapid heating and cooling of the glass surface d. Laminated glass = two sheets of ordinary glass bonded together with a plastic film i. Most car windshields e. Glass will possess its greatest evidential value when it can be individualized to one source. This can only be accomplished when pieces can be assembled and physically fitted together f. At this time, the physical properties of density and refractive index are used most successfully for characterizing glass particles g. Glass can be distinguished by a density determination and refractive indices. However, even glass fragments removed from a single sheet of glass may not have a uniform refractive index value h. A significant difference in either density or refractive index proves that the glasses examined do not have a common origin i. FBI has a database of density and refractive indices of different glasses collected Glass Fractures a. Glass bends in response to any force that is exerted on any one of its surfaces; when the limit of its elasticity is reached, the glass fractures b. Fractured glass may reveal information regarding force and direction of an impact c. Types of fractures i. Radial – a crack in a glass that extends outward like the spoke of a wheel from the point at which the glass was struck VI. ii. Concentric – a crack in a glass that forms a rough circle around the point of impact d. Stress marks – are shaped like arches that are perpendicular to one glass surface and curved nearly parallel to the opposite surface e. Radial cracks form a right angle on the reverse side of the force f. When there have been successive penetrations of glass, it is frequently possible to determine the sequence of impact by observing the existing fracture lines and their points of termination. A fracture always terminates at an existing line of fracture. Forensic Characteristics of Soil a. Soil = includes any disintegrated surface material, both natural and articial, that lies on or near the earth’s surface i. Encompasses analysis of naturally occurring material such as rocks, minerals, vegetation and animal matter as well as glass, plaint chips, asphalt, brick fragments, cinders etc. b. Value of soil as evidence rests with its prevalence at crime scenes and its transferability between the scene and the criminal c. Soil evidence is comparative in nature d. Most soils can be differentiated and distinguished by their gross appearance. Samples must be dried before comparison. e. Low power microscopic examination of soil will reveal the presence of plant and animal materials as well as of artificial debris f. Minerals = a naturally occurring crystal and like any other crystal, its physical properties are most useful for identification g. Some forensic laboratories currently rely on the density-gradient tube technique to compare soil specimens i. Density- gradient tube = a glass tube filled from bottom to top with liquids of successively lighter densities, used to determine the density distribution of soil h. The ultimate forensic value of soil evidence depends on its variation at the crime scene i. Up to this time, there have been no statistically valid forensic studies on the variability of soil evidence