* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PICORNAVIRIDAE
Herpes simplex wikipedia , lookup
2015–16 Zika virus epidemic wikipedia , lookup
Schistosomiasis wikipedia , lookup
Trichinosis wikipedia , lookup
Whooping cough wikipedia , lookup
Ebola virus disease wikipedia , lookup
Poliomyelitis eradication wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Oesophagostomum wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Influenza A virus wikipedia , lookup
Hepatitis C wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
West Nile fever wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Neonatal infection wikipedia , lookup
Marburg virus disease wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Herpes simplex virus wikipedia , lookup
Orthohantavirus wikipedia , lookup
Hepatitis B wikipedia , lookup
Henipavirus wikipedia , lookup
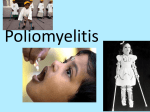
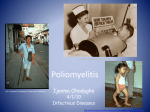
PICORNAVIRI DAE By Adey Berhanu Source: http://www.virology.net/Big_Virology/EM/polio1.gif Introduction Characteristics of Picornaviruses Genera Replication Epidemiology History Viral Profiles Polio Rhinoviruses New Findings Drug Profile Links Resources INTRODUCTION CHARACTERISTICS OF PICORNAVIRUSES: The picorna viral family derives its name from the words: pico which means small, and rna which stands for ribonucleic acid. The root of its name is appropriate because the viruses in the picornaviridae family are small RNA viruses. The virions are approximately 30nm in diameter and the genomes are roughly 7.0-8.5 kilobases. These viruses are infectious and range in the seriousness of disease. The The viruses of the picornavirdae family are classified together by following characteristics. Picornaviruses are single-stranded, positive sense RNA viruses. The virions are non-enveloped with icosahedral morphology. The genome is monopartite and has internal ribosome entry sites (IRES). Translation and cleavage result in 11 or 12 different proteins as products. GENERA: The members of the picornaviridae are classified in 5 genera, four of which can cause disease in humans. - Enteroviruses polioviruses 1-3, Human coxsackiviruses, Human echoviruses - Rhinoviruses Human rhinoviruses 1-103 - Hepatoviruses Human hepatitis A virus - Parechoviruses Human parechoviruses 1-2 - Aphtoviruses Infects animals such as cattle, goats, pigs, sheep, and rarely humans - Cardioviruses Infects rodents and rarely humans Source: http://images.google.com/images?q=tbn:Qi9bq_I8S2jVM:http://www.qub.ac.uk/afs/vs/vsd16d.jpg REPLICATION: The replication cycle can be summarized in the following steps. 1. Virus binds to cell receptor and the genome is uncoated. 2. The virion protein is removed and the viral RNA is translated. 3. The polyprotein is cleaved to produce distinct viral proteins. 4. Viral RNA polymerase copied to produce the negativesense RNA strand. 5. The negative-sense RNA strands are ultimately copied to produce more positive-sensed RNA strands. 6. In later infection, the positive-sense RNA strands enter the morphogenetic pathway. 7. Cell lysis occurs releasing newly synthesized virus particles. EPIDEMIOLOGY: For the most part, the picornaviruses have world wide distribution. Transmission most commonly occurs via the fecal-oral route or respiratory route depending on the specific virus. HISTORY: - In 1898, the first animal virus to be discovered was the foot-and-mouth disease virus which is a picornavirus. - The poliovirus was first isolated and identified in 1908. VIRAL PROFILES POLIO Source: http://www.ecbt.org/images/PolioL.jpg As a member of the enterovirus genus, polio can be considered among the most important. Beginning with the epidemics in the early 1800s, polio has presented itself as a serious public health issue. The poliovirus has three serotypes: poliovirus 13. HISTORY: - 2000 B.C.: Egyptian depictions of a young man with an atrophied limb believed to represent poliomyelitis. - 1800s: First clinical descriptions of poliomyelitis including cases of paralysis with fever. - 1952: Polio epidemic in the United States peaked with over 21,000 paralytic cases. - 1955: The Salk vaccine licensed in the United States. - 1961-1962: The Sabin vaccine licensed in the United States. - 1988: The World Health Organization launched a global polio eradication campaign EPIDEMIOLOGY: The poliovirus is considered to be highly infectious. Humans are the only known reservoir for the poliovirus. Transmission of the poliovirus is via the fecal-oral route. In temperate climates poliovirus infection commonly peaks during the summer months. The poliovirus has world wide distribution, however, since the advent of the vaccine poliovirus infection is extremely low in developed countries and cases are now highly concentrated in developing countries. PATHOGENESIS: The virus enters the body via the alimentary canal. Primary replication of the virus takes place in the throat (pharynx) and gastrointestinal tract. During viral shedding, before the onset of illness, the virus can be found in the throat and feces. The virus then invades the blood stream where it can infect the lymph tissue and possibly cells of the central nervous system. In the case that the poliovirus infects and replicates within the cells of the CNS such as the motor neurons of the brain stem, the resulting destruction is a manifestation of poliomyelitis. The polioviruses have a tropism for the cells of the central nervous system. CLINICAL MANIFESTATIONS: The incubation period for the polioviruses can be between 4 to 35 days though in most cases it is between 7-14 days. Most infections are asymptomatic or inapparent. The following clinical manifestations of disease can be divided into three categories: abortive poliomyelitis, aseptic meningitis, and paralytic poliomyelitis. Abortive poliomyelitis: This is a mild, non-specific illness. Manifestation of disease is characterized by a 2-3 day fever and influenza-like symptoms. There are no signs of CNS localization and complete recovery can be expected in less than a week. Aseptic meningitis: Symptoms include stiffness of the neck, back, and/or legs and last from 2 to 10days. Recovery is rapid and complete. Paralytic poliomyelitis: Begins with 2 to 3 days of minor illness such as fever and influenza-like symptoms. After several days these symptoms disappear and return within 5 to 10 days along with signs of meningeal irritation. Flaccid paralysis ensues characterized by cramping muscle pain, spasms, coarse twitching, and tendon reflexes are diminished. Encephalitis is rare but possible. Patients do not show loss of sense or cognitive changes. Recovery is possible however paralysis lasting beyond 6 months is permanent. TREATMENT & PREVENTION Treatment of polio is supportive. There is no effective antiviral therapy available. Prevention of polio is possible via vaccination. There are two licensed vaccines in the United States: the Salk vaccine (IPV) and the Sabin vaccine (OPV), but only one is used. -The Sabin vaccine, a live-attenuated virus, distributed orally (OPV) was discontinued in 2000 due to its association with causing paralytic disease. Source: http://upload.wikimedia.org/wikipedia/en/9/9b/Stamp-ctc-polio-vaccine.jpg - The Salk vaccine, an inactivated poliovirus vaccine, (IPV) is recommended to prevent against polio. IPV contains all three serotypes of poliovirus and is administered intramuscularly. IPV is considered safe with no significant side effects. IPV is part of the United States vaccination schedule of children. Primary vaccination with three subcutaneous doses is recommended for unimmunized children and adults. A booster dose is recommended for children. Protection is believed to be a duration of 5 years, possibly longer. -In 1988 the World Health Organization launched a global polio eradication campaign and it still remains one of the organization’s priorities. RHINOVIRUS Currently, there are 103 different rhinovirus serotypes. EPIDEMIOLOGY: Rhinoviruses have world wide distribution and are extremely common. Rhinoviruses are transmitted via aerosol droplets—virus-contaminated respiratory secretions. CLINICAL MANIFESTATIONS: Rhinoviruses are the most frequent cause of the “common cold.” The incubation period is between 1 to 4 days. The illness caused by rhinovirus is mild and typical symptoms include nasal discharge, nasal secretions, sneezing, and sore throat. In more serious cases fever and upper respiratory infection are possible. Illness usually resolves itself within a week. TREATMENT & PREVENTION -There are currently no antiviral therapies available. However, over-the-counter cold remedies are useful in relieving the symptoms caused by rhinovirus. -No effective vaccine is available. NEW FINDINGS Implications of Cosxackie B virus infection and pancreatitis This article presents the result of a study investing the differences in morbidity and mortality associated with the different routes of infection of the Coxsackie B virus. Swiss albino mice were infected with either oral or intraperitoneal (into the abdominal cavity) injection of Coxsackie B virus. The results showed that virus titres were higher in intraperitoneal infection versus oral infection. Both routes showed infection of the pancreas, however, only intraperitoneal injection showed damage to the pancreas. In both routes of transmission the pancreatic islets were spared and viral VP1, protein 3A, and alpha interferon could be found. The article concludes that the oral route of infection restrains the pathology in the pancreas. Second, the Coxsackie B virus is associated with the prolonged presence of alpha interferon. Therefore, the oral route of infection may imply a natural way of investigating the relationship of enteroviruses such as Coxsackie B virus in pancreatitis and type 1 diabetes mellitus. -Bopegamage, Shubhada, et al. “Coxsackie B virus infection of mice: inoculation by the oral route protects the pancreas from damage, but not from infection.” The Journal of general virology. (86): 2005. pg:3271 3280. Human Parechovirus 3 and neonatal infections This article investigates the details concerning the third serotype of the human parechovirus that was recently isolated in Japan infants. The article investigates 3 additional cases of neonatal sepsis human parechovirus 3 in Canadian newborns. The three cases were similar in that the infants were hospitalized with high fever, erythematous rash, tachypnea for several days. Viruses removed from the nasopharyngeal tissue had viral proteins closely related to three serotypes of the human parechovirus. Therefore, this report presents three other confirmed cases of human parechovirus outside of Japan and the first description of neonatal sepsis caused by human parechovirus serotype 3. - Boivin, Guy et al. “Human parechovirus 3 and neonatal infections.” Emerging Infectious Diseases. (11): 1, Jan 2005. pg. 103-105. Rhinoviruses and asthma This article discusses how rhinoviruses may be associated with 60% of the viral, upper respiratory tract infections involved in asthma exacerbations. It is believed that the rhinovirus has specific pathological activity that results in the relapse of acute asthma. Rhinovirus produces a reactivation of asthmatic symptoms as well as a deterioration of respiratory function and an increase in bronchial hyper-responsiveness. The cytotoxic effect of the rhinovirus is believed to facilitate the penetration of allergens. Therefore, through several mechanisms the rhinoviruses have associated with triggering or exacerbating asthma. - Pelaia, Girolamo, et al. “Respiratory infections and asthma.” Respiratory Medicine. Nov 2005. doi:10.1016/j.physletb.2003.10.071. Echoviruses interaction with DAF This article analysis how the Echoviruses bind to the Decay Accelerating Factor (DAF) in order to facilitate entry of the virus into the cell. In this specific article uses cryo-negative stain transmission electron microscopy and three-dimension image reconstruction to investigate the binding of echoviruses type 7, 11 and 12 to DAF. Results indicate that the differences in interactions of the different serotypes is due to small subtleties in structure rather than in large scale repositioning of the virus surface. - Pettigrew, David, et al. “The Interactions of Echoviruses and Decay Accelerating Factor (DAF): Structural and Functional Insights.” The Journal of Biological Chemistry. 7 Nov 2005. M510362200. Recombinant poliovirus-SIV cocktails and HIV research The article proposes the use of the Sabin poliovirus vaccine to research effective targets for HIV medications. Based on the Sabin poliovirus vaccine, researchers have created replicationcompetent poliovirus recombinants that express antigens derived from the simian immunodeficiency virus (SIV). This recombinant poliovaccine is then injected into mucosal tissue to observe the cellular immunity that is induced. This recombinant poliovirus-SIV vaccine has demonstrated protection against HIV infection and AIDS in cynomolgus monkeys. - Crotty, Shane and Raul Andino. “Poliovirus vaccine strains as mucosal vaccine vectors and their potential use to develop an AIDS vaccine.” Advanced Drug Delivery Reviews. (56): 6. 19 April 2004. Pg. 835-852. DRUG PROFILE Ganciclovir Source: http://virology-online.com/general/ganciclovir.gif Gangciclovir, 9-[(1,3-dihydroxy-2propoxy)methyl]guanine Brand name: Cytovene Ganciclovir is an antiviral drug. Ganciclovir is an analogue of acyclovir. Mechanism: Ganciclovir acts by inhibiting viral DNA polymerase and can be incorporated into viral genome where it eventually terminates DNA elongation. Use: Ganciclovir is used primarily to treat patients with Cytomegalovirus (CMV) infection of the eyes. However, this antiviral is also active against all herpes infections. Administration: Gancliclovir can only be administered intravenously. Dosage: Initial therapy is 5mg/kg twice a day for 14 to 21 days followed by a dose of 5mg/kg five times a week. Side effects: headache, diarrhea, nausea, vomiting, upset stomach, loss of appetite, dizziness. Also, associated with profound bone marrow suppression, particularly neutropenia. LINKS - Center for Disease Control and Prevention: http://www.cdc.gov/ - National Institute of Health: http://www.nih.gov/ - Polio: http://www.cdc.gov/nip/publications/pink/polio.pdf - Enteroviruses: http://www.cdc.gov/ncidod/dvrd/revb/enterovirus/n on-polio_entero.htm - Hepatitis A: http://www.cdc.gov/ncidod/diseases/hepatitis/a/ind ex.htm RESOURCES: Boivin, Guy et al. “Human parechovirus 3 and neonatal infections.” Emerging Infectious Diseases. (11): 1, Jan 2005. pg. 103-105. Bopegamage, Shubhada, et al. “Coxsackie B virus infection of mice: inoculation by the oral route protects the pancreas from damage, but not from infection.” The Journal of general virology. (86)2005. pg:3271 -3280. Crotty, Shane and Raul Andino. “Poliovirus vaccine strains as mucosal vaccine vectors and their potential use to develop an AIDS vaccine.” Advanced Drug Delivery Reviews. (56): 6. 19 April 2004. Pg. 835-852. Isselbacher, Kurt J, et al. Harrison’s Principles of Internal Medicine. 13th edition. New York: McGraw-Hill, Inc, 1994. Knipe, David, et al. “Picornaviridae.” Fields Virology. 4th ed. Lippincott Williams & Wilkins: Philadelphia, 2001. Pelaia, Girolamo, et al. “Respiratory infections and asthma.” Respiratory Medicine. Nov 2005. doi:10.1016/j.physletb.2003.10.071. Pettigrew, David, et al. “The Interactions of Echoviruses and Decay Accelerating Factor (DAF): Structural and Functional Insights.” The Journal of Biological Chemistry. 7 Nov 2005. M510362200. “Poliomyelitis.” Center for Disease Control and Prevention. <http://www.cdc.gov/nip/publications/pink/polio.pdf>