* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Unit 1 Density and Connections PowerPoint
Survey
Document related concepts
Transcript
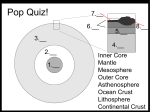
Ice Density: 0.9 g/mL Water Density: 1.0 g/mL UNIT 1: DENSITY AND CONNECTIONS Granite Density: 2.7 g/mL Basalt Density: 3.0 g/mL After Unit 1 you should be able to: o Understand how to make observations and develop inferences in Earth Science o Calculate density using the proper units o Work interchangeably within the density equation to determine mass or volume of a substance o Understand that density describes how much matter is in a given volume of a solid, liquid, or gas o Understand the properties of volume and mass o Understand how substances in a mixture behave when they have unique densities o Understand that heat expands the volume of a substance and decreases the density, and that cooling a substance decreases the volume and increases the density o Navigate the reference tables that use density in some way o Calculate volume and use the water displacement method o Convert milliliters to cubic centimeters o Understand that density of a uniform substance does not change regardless of size o Understand how convection currents form o Understand the special circumstance surrounding water and density Unit 1 vocabulary you should be able to use and understand: o o o o o o o o o o o o o o o o o o o Formula Mass Volume Density Grams Milliliter Centimeter Cubic centimeter Electronic balance Water displacement Ratio Matter Contract Expand Crust Tectonic plates Continental crust Oceanic crust Convergent boundary o o o o o o o Observation Inference Triple Beam Balance Elevation Jovian planets Terrestrial planets Convection currents The scientific method is driven by a balance between making accurate observations and developing reasonable inferences from those observations. This course requires you to think with a scientific mind. Being able to develop sound, evidencebased inferences from observations made in Earth Science will ensure your success. Making Observations Observations are made using the five senses: Sight Touch Taste Smell Hearing Of these five, the sense of sight is the most commonly relied upon sense in Earth Science investigations. Observations often require the use of scientific instruments to be accurate. A scientific instrument is any tool that you can use to make measurements or additional observations. Examples: hand lens, electronic balance, triple-beam balance, graduated cylinder Making Inferences An inference is an educated guess or conclusion derived from one or more observations For example: A student discovers a very large boulder in upstate New York that does not match local bedrock in the area. An inference could be that the boulder was transported by glaciers. The student observed the differences in bedrock, but did not see glaciers transport the rock, however there is supporting evidence for glaciers covering the area. Density is a property of matter that is the ratio of mass to volume of a substance. Understanding how density impacts the behavior of interacting substances is critical in Earth Science. The Equation in the Earth Science Reference Tables Density = mass/volume Before we get into the equation as a whole, let’s consider the components: mass and volume What is mass? Mass is the measure of matter that makes up an object. It is very similar to weight but is not the same. Object with a high mass: an anvil, containing iron Object with a low mass: a feather What is volume? Volume is the amount of space an object takes up. Container with a large volume: water tower Container with a low volume: water bottle How do you find an object’s mass? Place the object on an electronic balance or triple beam balance A triple beam balance requires you to find the sum of the mass of all three bars How can you determine an object’s volume? If the object is a regular shape, like a rectangular prism, the volume can be determined mathematically by performing the following calculation (length x width x height) When performing such a calculation, centimeters are used. When multiplied (#cm x #cm x #cm), the appropriate units are cm3. How can you determine an object’s volume? The second method is water displacement. Measure the preexisting amount of water in a container, then gently place the sample in the water. Record the difference between the new measurement and old measurement. This value is the volume of the sample in milliliters (mL). 1 mL is equal to 1 cm3, so these units may be used interchangeably, although milliliters are commonly used for liquids or irregular samples. Before finally diving into density, let’s consider our earlier examples for understanding mass. Which is heavier, a pound of iron from the anvil, or a pound of feathers? A pound of which material would take up the most volume? Let’s look back at the equation: Density = mass/volume Density is simply the ratio of the amount of mass (matter) inside the space of an object So in our feathers and anvil example, we can say that the feathers are much less dense than the iron from the anvil. Sample Problem: An Earth Science student is trying to determine the density of a sample of basalt she discovered in the field. The sample has a mass of 60 grams and a volume of 20 mL. What is the density of the sample? Density = mass/volume Solution: ___________ g/mL What if an object is cut in half? Does the density change? A student has a wooden block that has a mass of 36 grams and a volume of 48 cm3. The density is 0.75 g/cm3 After cutting the block in half, the mass is now 18 grams, and the volume is 24 cm3. The density is 0.75 g/cm3 Density remains the same in the same material, no matter the sample size. How can the density of a substance change? Density will decrease if you increase the volume of an object (expansion). Most materials decrease in density by increasing in volume during heating. If the material cools, it will contract and become more dense. What do less dense materials tend to do when mixed with more dense materials? Less dense materials rise! More dense materials sink! In liquids and gases, this results in the formation of convection currents, a transference of energy upward due to differences in density. Convection commonly takes place in the atmosphere and in a layer of the Earth known as the asthenosphere. Things to know about density in Earth Science: 1. The Earth is layered based on density, with more dense materials in the core, pulled there by gravity 2. Planets closest to the Sun (Terrestrial: Mercury, Venus, Earth, Mars) are more dense than ones farther away (Jovian: Jupiter, Saturn, Neptune, Uranus) 3. When air warms, it will expand and become less dense, and rise More things to know about density in Earth Science: 4. When air cools it will contract and become more dense, and sink 5. Earth’s crust is made up of plates of varying densities. More dense plates (oceanic crust, made of basalt) plunge below thicker, but less dense plates (continental crust, made of granite) at convergent boundaries. 6. The density of liquid water is 1.o g/mL at about 4 oC, and it decreases as it freezes to ice. 7. Convective circulation caused by #’s 3 and 4 results in hurricane, monsoon, and land and sea breeze formation (covered in Unit 12) Relevant Reference Tables for Density