* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Experiment 4 Separation of a Mixture
Survey
Document related concepts
Transcript
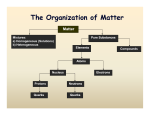
Experiment 4 Separation of a Mixture OBJECTIVES To learn how to use physical properties of four different substances to design a scheme for the separation of a mixture. After the separation is achieved,, percent composition of each component in the mixture will be calculated. DISCUSSION One of the most important laboratory techniques used by chemists is called separation. Often when chemists encounter an unknown mixture, their first objective is to separate and then identify the mixture into its constituent parts. But before we go on we need to first define the difference between an element, compound, and a mixture. Element: An element is a fundamental component of matter that can not be broken down by simple chemical means. The periodic table, which we have talked about in class, contains all the elements we know about up to this point. An example of an element is copper (Cu). At room temperature copper is a solid, if the temperature is raised above the melting point of copper it becomes a liquid. But the element has not changed; it is still made up of copper atoms. Compound: Substances that are made up of elements and can be broken down into different elemental parts only by chemical means. A good example of a compound is the metal brass. This soft metal is made up of different amounts of copper (Cu), zinc (Zn), tin (Sn), lead (Pb), and iron (Fe). A brass block can not be physically separated into these elemental parts, it will take chemistry to be able to do that. Mixture: A combination of elements and /or compounds that do not react chemically and can be separated by physical means. If a beaker contains a mixture of copper (Cu) and iron (Fe) powder at room temperature these two elements can be separated. It might take a while, but it can be done. Any suggestions on how you might do that? Given an unknown mixture, a chemist knows that each of its components has their own fundamental physical properties that enable us to separate and identify these individual components and determine their percent composition. Some of the physical properties that we will use in this lab to separate a mixture includes solubility, boiling point, and sublimation. The solubility of a substance indicates how easily a particular substance called a solute dissolves in a solvent, which is often a liquid. The boiling point is the temperature at which a substance changes from the liquid phase to the vapor phase. Finally, sublimation is the ability of a substance to change from the solid phase to the gas phase without ever entering the liquid phase. This doesn’t happen for many solids at room temperature, which will help us in designing our separation procedure. This leads to the different types of separations that are commonly used by chemist: 1- Distillation is the process of heating the liquid to its boiling point, condensing it (by cooling), and collecting the vapors. This process uses the differing boiling points of liquids for separation. 2- Extraction and Filtration utilizes the solubility of a substance to permit separation. Extraction is the process of removing a substance with a greater solubility from a substance with a lower solubility. Filtration is the process of straining a solid suspended in a liquid. 3- Centrifugation is the process of separation a suspended solid in a liquid from the liquid by high speed rotation. 4- Chromatography is the process by which substances pass through a stationary phase (different type of molecules) and are separated by difference in physical attraction to this phase. We will talk more about these methods later in the quarter. In this experiment you will be given a mixture of iron filings (Fe), ammonium chloride (NH4Cl), sodium chloride (NaCl), and sand which is silicon dioxide (SiO2) of unknown composition (you will not know how much of each substance is present in the solid mixture). By carefully examining the table below, which details the different physical properties of the species you will be analyzing, you will then design a separation scheme for your mixture. Table of Physical Properties Sodium chloride CHEMICAL Ammonium chloride NaCl NH4Cl 58.5 53.5 Silicon dioxide SiO2 Iron Fe EQUATION MOLECULAR 60.1 55.8 WEIGHT SOLUBILITY (g ) in 100g water at 25 C MELTING POINT C 35 37 801 sublimes 350 insoluble 1600 insoluble 1535 white APPEARANCE white white crystals crystals crystals grains dark brown We will at the beginning of lab talked extensively about the different separation techniques and the above table, after which time you will be asked to design a flow chart that will be used to separate the different components of an unknown mixture. Your flow chart will be rather general to begin with since the detailed steps of the separation techniques are, as of yet, unknown to you—we will discuss that in class. But from examining the table you should be able to logically derive a procedure to determine the percent composition of sand, salt, iron, and ammonium chloride. You will be working with a partner in this lab. Carefully look at the chart and try and find unique physical properties that could be use to separate these different components. If you have any questions please ask the instructor. After you have designed your procedure in the form of a flow chart show it to your instructor for final approval. Once your procedure has been approved the instructor will help you figure out the detailed procedure with the chemical techniques to be use in this experiment. Once the different components have been isolated you will weigh each of them and find the percent composition of each in the unknown mixture. After this has been completed, ask the instructor for the real percentage values for each. This comparison will be use then to discuss the possible errors involved in the experiment and how your procedure could have been improved. Pre-laboratory Assignment Separation of Mixtures Experiment#4: 1. Give an example of an element involved in this lab. 2. One of the components to be separated is salt. What type of chemical bond is found in salt? 3. Define solubility. Is salt very soluble in water? 4. You could go to an alternative food store and buy "Sea Salt" for 5 times what it cost to by regular table salt. But you could also gather it yourself. Briefly describe how this could be done.