* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Matter Exam Study Guide
Equilibrium chemistry wikipedia , lookup
Degenerate matter wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Chemical imaging wikipedia , lookup
Atomic theory wikipedia , lookup
Chemical potential wikipedia , lookup
Physical organic chemistry wikipedia , lookup
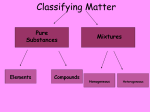
Matter Exam Study Guide Directions: Complete the study guide to receive 5 bonus points on your exam. This must be turned in complete for credit. Please put your answers on a separate piece of paper. 1. What is the definition of matter? 2. What is the definition of mass? 3. What is the definition of volume? 4. What is the definition of density? 5. What are the four states of matter discussed in this class? 6. Define a solid. 7. Define a Liquid. 8. Define a gas. 9. Describe how the molecules of a gas are. 10. Describe how the molecules of a liquid behave. 11. Describe how the molecules of a solid behave. 12. What is an atom? 13. What is the difference between a molecule and a compound? 14. What is an element? Be able to recognize an element by its symbol (He, H, O, C, Na, etc). 15. What is a pure substance? What is an example of a pure substance? 16. What is a mixture? Give an example. 17. What is a homogenous mixture? 18. What is a heterogeneous mixture? 19. What is a physical property? What are three examples of physical properties? 20. What is a chemical property? What are three examples of chemical properties? 21. What is a physical change? Give three examples. 22. What is a chemical change? Give three examples. 23. What is an extensive property? Give an example. 24. What is an intensive property? Give an example. 25. What is the Law of Conservation of Matter? What does that mean? 26. What are some signs that a chemical change has occurred? 27. What is a solution? 28. What is a solvent? What is a solute? 29. What is an alloy? 30. Why do chemical equations need to be balanced? 31. Balance the following chemical equations: N2 + H2 → NH3 C3H8 + O2 → CO2 + H2O