* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Metaphylaxis of healthy in contact animals to replace
Tuberculosis wikipedia , lookup
Neglected tropical diseases wikipedia , lookup
Trichinosis wikipedia , lookup
Oesophagostomum wikipedia , lookup
Schistosomiasis wikipedia , lookup
Marburg virus disease wikipedia , lookup
Traveler's diarrhea wikipedia , lookup
Onchocerciasis wikipedia , lookup
Brucellosis wikipedia , lookup
Leishmaniasis wikipedia , lookup
Lymphocytic choriomeningitis wikipedia , lookup
Fasciolosis wikipedia , lookup
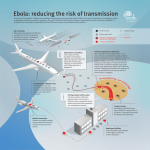
“Metaphylaxis” of healthy ‘in contact’ animals to replace “prevention” in an infected environment From now on, “metaphylaxis” is a term recognised by the European Medicines Agency (EMA). It refers to the treatment of clinically healthy (but presumably infected) in-contact animals. However, it does not include the treatment of animals on premises that are presumably infected due to the former (not contemporary) presence of sick animals. From now on, the European Medicines Agency for human and veterinary health recognises two possible uses for veterinary antibiotics: “treatment” and “metaphylaxis”. The word “prevention” in combination with “treatment” is to be replaced by the word “metaphylaxis” in the SPCs (summary of product characteristics) of the approved antibiotics. Definitions for 2015 The European definitions for 2015 are as follows. 1. The term “treatment” refers to the treatment of an individual animal, or a group of animals showing clinical signs of an infectious disease. This implies curative treatment only. 2. The term “metaphylaxis” refers to the administration of the product at the same time to a group of clinically healthy (but presumably infected) in-contact animals, to prevent them from developing clinical signs, and to prevent further spread of the disease. The presence of the disease in the group/flock must be established before the product is used. A number of antibiotics have indications containing the expression “for treatment and prevention”. In the mid- to long-term, this expression is to be replaced by “treatment and metaphylaxis”. The EMA recalls that a metaphylaxis claim will always have to be combined with a treatment claim. The word “prevention” open to interpretation In 2011, the EMA underlined that the term “prevention” when used for an antimicrobial product should not be understood as “routine preventive use on healthy animals”. However, a number of antimicrobial products for food-producing species have indications containing the expression “prevention” or “preventive treatment” against infections. It is clear that the word “prevention” is open to a wide range of interpretations and translations, leading to differences in the indications for use in the SPCs for the same product in different countries/languages. The EMA is concerned that no difference is made between vaccines and antibiotics regarding the “prevention” of disease in healthy animals. In France: “prevention in an infected environment” French authorities have accepted the term “prevention in an infected environment” in the data sheets. This includes the “prevention” of disease in healthy in-contact animals, or “metaphylaxis” in English. However, “prevention in an infected environment” also includes the protection of healthy animals against clinical disease on presumably infected premises, where a pathogen was detected in an earlier batch of animals. Metaphylaxis of in-contact animals However, metaphylaxis does not include the prevention of disease in healthy animals on presumably infected premises since the sick animals were in a previous batch. It only refers to the treatment of healthy but presumably infected in-contact animals – and not to those that were presumably infected because they were housed in barns in which sick animals had been housed in earlier batches. The benefit of metaphylaxis to be proven Regarding clinical trials of new antibiotics, the EMA should be quite strict when accepting the claim of metaphylaxis. Such trials should prove that metaphylaxis presents a clear benefit over the treatment of individual animals or a group of animals showing clinical signs of disease. Quite a challenge. Source: European Medicines Agency (EMA). Question and answer on the CVMP guideline on the SPC for antimicrobial products (EMEA/CVMP/SAGAM/383441/2005). EMA/CVMP/414812/2011-Rev.1 http://www.ema.europa.eu/docs/en_GB/document_library/Other/2011/07/WC500109155.pdf