* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 16-1 and 16-2 Electric Charge
Speed of gravity wikipedia , lookup
Electromagnetism wikipedia , lookup
Maxwell's equations wikipedia , lookup
Lorentz force wikipedia , lookup
History of electromagnetic theory wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Atomic theory wikipedia , lookup
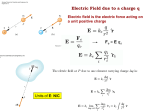
Chapter 16 Electric Charge and Electric Field Units of Chapter 16 • Static Electricity; Electric Charge and Its Conservation • Electric Charge in the Atom • Insulators and Conductors • Induced Charge; the Electroscope • Coulomb’s Law • Solving Problems Involving Coulomb’s Law and Vectors • The Electric Field Units of Chapter 16 • Field Lines • Electric Fields and Conductors Objectives: The students will be able to: 1. Demonstrate that charged objects exert forces, both attractive and repulsive. 2. Explain that charging is the separation, not the creation, of electric charge. Objectives: The students will be able to: 1. State from memory the magnitude and sign of the charge on an electron and proton and also state the mass of each particle. 2. Apply Coulomb's law to determine the magnitude of the electrical force between point charges separated by a distance r and state whether the force will be one of attraction or repulsion. 3. State from memory the law of conservation of charge. 4. Distinguish between an insulator, a conductor, and a semi conductor and give examples of each. 5. Explain the concept of electric field and determine the resultant electric field at a point some distance from two or more point charges. 6. Determine the magnitude and direction of the electric force on a charged particle placed in an electric field. 7. Sketch the electric field pattern in the region between charged objects. 8. Use Gauss's law to determine the magnitude of the electric field in problems where static electric charge is distributed on a surface Electricity • Hewitt’s intro to Electricity Static electricity Static electricity is caused when certain materials are rubbed against each other. Electrons can be rubbed off one material and on to another. The material that has got extra electrons is now negatively charged The material which has lost electrons is positively charged. - + - + - + + - - - + + + - + + + - - + - + - + + - - - + + + - + + + - - + - + - + + - - - + + + - + + + - - + - + - + + - - - + + + - + + + - - + - + - + + - - - + + + - + + + - - + - + - + - + - + + + - + + + - 16.1 Static Electricity; Electric Charge and Its Conservation Objects can be charged by rubbing Static Electricity Static electricity is the electric charge at rest on an object. When something is static, it is not moving. The charges of static electricity do not move away from the object that they are in. So, the object keeps its charge. Ex. Clothes taken out of a dryer Static electricity It is this imbalance of positive and negative charges that causes: Balloons to stick to walls. Your hair to stand on end when brush your hair on a dry day. And the electric shock you sometimes get from the door handle. Exploring Electric Charge phET site Electric Discharge The loss of static electricity as charges move off an object is called electric discharge. Sometimes, electric Sometimes discharge , electric happens discharge quickly. happens slowly. Ex. wearing Ex: static on clothes rubber-soled shoes on carpet, lightning Static Discharge Human body can not feel less than 2,000 volts of static discharge Static charge built up by scuffing shoes on a carpet can exceed 20,000 volts? Gas Station Fires • Carol said a static gas pump fire is blamed for burning her daughter so badly she needed skin grafts on her legs. • Carol had put the gas pump nozzle on automatic and re-entered her car to write a check. When her then-12-year-old daughter, wearing a sweater and jacket that may have created static electricity, reached for the nozzle, flames suddenly ignited her clothing. Grounding What is grounding? An object is grounded when it is connected to the earth through a connecting wire. If a charged conductor is grounded, it will become neutral. Grounding b How does grounding occur? + + + + + When we touch a metal ball of positive charge... electrons flow from the earth to the metal ball to neutralize the metal ball. Metal ball becomes neutral. Grounding How does grounding occur? – – – – – Similarly, if the metal ball is of negative charge... extra electrons flow from the metal ball to the earth and the ball becomes neutral. Why do gasoline tankers usually have metal chains at the back? When cars run, their tires and bodies are usually friction For gasoline tankers, if the charged by _______. accumulated charge is large enough, _______can be sparks explosion will occur if gasoline vapor is produced and _________ ignited. Those metal chains conduct the charge on the ground and avoid the danger. bodies of tankers to the _______ 16.1 Static Electricity; Electric Charge and Its Conservation Charge comes in two types, positive and negative; like charges repel and opposite charges attract 16.1 Static Electricity; Electric Charge and Its Conservation Electric charge is conserved – the arithmetic sum of the total charge cannot change in any interaction. Electric Charge • All matter is made up of atoms • 1. 2. 3. Atoms contain Protons (+) Neutrons (0) Electrons (-) 16.2 Electric Charge in the Atom Atom: Nucleus (small, massive, positive charge) Electron cloud (large, very low density, negative charge) 16.2 Electric Charge in the Atom Atom is electrically neutral. Rubbing charges objects by moving electrons from one to the other. Where do charges come from? If electrons = protons neutral If electrons > protons gaining electrons, negative charge If electrons < protons losing electrons, positive charge Electro-negativity +++++ ++++ +++ ++ + Relative electro-negativity ranking for some common materials from electron donating materials (+, glass) to -electron accepting --materials (-, teflon) -------- • • • • • • • • • • • • Glass Human Hair Nylon Silk Fur Aluminum Paper Cotton Copper Rubber PVC Teflon Where do charges come from? Rubbing materials does NOT create electric charges. It just transfers electrons from one material to the other. Where do charges come from? When a balloon rubs a piece of wool... + – – – – – – wool + + + + electrons are pulled from the wool to the balloon. The balloon has more electrons than usual. The balloon: – charged, The wool: +charged 16.2 Electric Charge in the Atom Polar molecule: neutral overall, but charge not evenly distributed Law of Electric Charges • The law of electric charges states that like charges repel, and opposite charges attract. • Protons are positively charged and electrons are negatively charged, so they are attracted to each other. • Without this attraction, electrons would not be held in atoms. Law of Electric Charges Elaboration • Hand-out – Rule of Electric Charges Homework for 16-1 and 16-2 Questions on page 464 #s 2, 3, 4, 5 Closure Kahoot 16-1 and 16-2