* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ppt
Elementary particle wikipedia , lookup
Anti-gravity wikipedia , lookup
Valley of stability wikipedia , lookup
History of subatomic physics wikipedia , lookup
Nuclear fission product wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear force wikipedia , lookup
Nuclear structure wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Nuclear drip line wikipedia , lookup
Nuclear fusion wikipedia , lookup
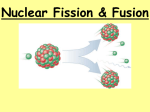
General Physics (PHY 2140) Lecture 20 Modern Physics Nuclear Energy and Elementary Particles Fission, Fusion and Reactors Elementary Particles Fundamental Forces Classification of Particles Conservation Laws Chapter Chapter 3030 http://www.physics.wayne.edu/~alan/2140Website/Main.htm Previously… Nuclear Physics Nuclear Reactions Medical Applications Radiation Detectors Review Problem: A beam of particles passes undeflected through crossed electric and magnetic fields. When the electric field is switched off, the beam splits up in several beams. This splitting is due to the particles in the beam having different A. masses. B. velocities. C. charges. D. some combination of the above E. none of the above v = E/B r=mv/qB Processes of Nuclear Energy Fission A nucleus of large mass number splits into two smaller nuclei Fusion Two light nuclei fuse to form a heavier nucleus Large amounts of energy are released in either case Processes of Nuclear Energy Fission A nucleus of large mass number splits into two smaller nuclei Fusion Two light nuclei fuse to form a heavier nucleus Large amounts of energy are released in either case Fission Fusion Nuclear Fission A heavy nucleus splits into two smaller nuclei The total mass of the products is less than the original mass of the heavy nucleus First observed in 1939 by Otto Hahn and Fritz Strassman following basic studies by Fermi Lisa Meitner and Otto Frisch soon explained what had happened Fission of 235U by a slow (low energy) neutron 236 n 235 U 92 92 U* X Y neutrons 1 0 236U* is an intermediate, short-lived state X and Y are called fission fragments Many combinations of X and Y satisfy the requirements of conservation of energy and charge Sequence of Events in Fission The 235U nucleus captures a thermal (slow-moving) neutron This capture results in the formation of 236U*, and the excess energy of this nucleus causes it to undergo violent oscillations The 236U* nucleus becomes highly elongated, and the force of repulsion between the protons tends to increase the distortion The nucleus splits into two fragments, emitting several neutrons in the process Energy in a Fission Process Binding energy for heavy nuclei is about 7.2 MeV per nucleon Binding energy for intermediate nuclei is about 8.2 MeV per nucleon Therefore, the fission fragments have less mass than the nucleons in the original nuclei This decrease in mass per nucleon appears as released energy in the fission event An estimate of the energy released Assume a total of 240 nucleons Releases about 1 MeV per nucleon 8.2 MeV – 7.2 MeV Total energy released is about 240 MeV This is very large compared to the amount of energy released in chemical processes QUICK QUIZ In the first atomic bomb, the energy released was equivalent to about 30 kilotons of TNT, where a ton of TNT releases an energy of 4.0 × 109 J. The amount of mass converted into energy in this event is nearest to: (a) 1 g, (b) 1 mg, (c) 1 g, (d) 1 kg, (e) 20 kilotons (c). The total energy released was E = (30 ×103 ton)(4.0 × 109 J/ton) = 1.2 × 1014 J. The mass equivalent of this quantity of energy is: E 1.2 1014 J 3 m 2 1.3 10 kg ~ 1g 8 2 c (3.0 10 m/s) Note: 1 gram TNT = 4184 J (exactly) Chain Reaction Neutrons are emitted when 235U undergoes fission These neutrons are then available to trigger fission in other nuclei This process is called a chain reaction If uncontrolled, a violent explosion can occur The principle behind the nuclear bomb, where 1 g of U can release energy equal to about 30000 tons of TNT 11 Mt H-bomb Nuclear Reactor A nuclear reactor is a system designed to maintain a self-sustained chain reaction The reproduction constant, K, is defined as the average number of neutrons from each fission event that will cause another fission event The maximum value of K from uranium fission is 2.5 Two 235U reactions, one yields 3 the other 2 neutrons In practice, K is less than this A self-sustained reaction has K = 1 Basic Reactor Design Fuel elements consist of enriched Cadmium uranium (a few % 235U rest 238U) The moderator material helps to slow down the neutrons The control rods absorb neutrons When K = 1, the reactor is said to be critical The chain reaction is selfsustaining When K < 1, the reactor is said to be subcritical The reaction dies out When K > 1, the reactor is said to be supercritical A run-away chain reaction occurs SCRAM = Safety Control Rod Axe Man D2O, graphite Schematic of a Fission Reactor Nuclear Fusion When two light nuclei combine to form a heavier nucleus Is exothermic for nuclei having a mass less than ~20 (Iron is the limit, Z=26, A=56) The sun is a large fusion reactor The sun balances gravity with fusion energy Sun’s Proton Cycle First steps: H + 11 H 2 1 H + e+ ν e H + 21 H 3 2 He + γ 1 1 1 1 2% of sun’s energyis carried by neutrinos Followed by H – He or He – He fusion: or 1 1 3 2 H + 23 He He + 23 He 4 2 He + e + ν e 4 2 He + 11 H + 11 H Total energy released is 25 MeV Net Result 4 protons (hydrogen nuclei) combine to give • • • • An alpha particle (a helium nucleus) Two positrons One or two neutrinos (they easily escape) Some gamma ray photons (absorbed) The two positrons combine with electrons to form more gamma photons The photons are usually absorbed and so they heat the sun (blackbody spectrum) Fusion Reactors Enormous energy in a small amount of fuel 0.06g of deuterium could be extracted from 1 gal of water This represents the equivalent energy of ~6x109 J Fusion reactor would most likely use deuterium and tritium 2 1 H + 21 H 3 2 He + 01 n, Q 3.27 MeV H + 21 H 31 H + 11 H, Q 4.03 MeV 2 3 4 1 H + H He + 1 1 2 0 n, Q 17.59 MeV 2 1 Advantages of fusion power Fuel costs are relatively small Few radioactive by-products of fusion reaction (mostly helium-3 and helium-4) Disadvantages of fusion power Hard to force two charged nuclei together Reactor is complex and expensive Need high temperatures and pressures to achieve fusion (~108 K) need a plasma Plasma confinement Plasma ion density, n Plasma confinement time, In order to achieve a fusion reaction need to satisfy Lawson’s criterion: n 10 s/cm 14 3 n 1016 s/cm3 Deuterium- tritium reactor Deuterium- deuterium reactor So need 108 K for 1 second Fusion Reactors - 1 Inertial confinement Inject fuel pellets and hit them with a laser (lots of lasers) or ion beams to heat them Imploding pellet compresses fuel to fusion densities Doesn’t require plasma confinement via magnetic fields Requires large facility to house lasers and target chamber. National Ignition Facility the facility is very large, the size of a sports stadium the target is very small, the size of a BBgun pellet the laser system is very powerful, equal to 1,000 times the electric generating power of the United States each laser pulse is very short, a few billionths of a second The beams are generated in the laser bay and deliverd to the target bay. The National Ignition Facility The target chamber Fusion Reactors - 2 Magnetic field confinement Tokamak design – a toroidal magnetic field First proposed by Russian scientists Fusion Reactors – cont. Tokamak Fusion Test Reactor – ITER International Thermonuclear Experimental Reactor To be constructed in Cadarache in the South of France. ITER’s proposed site layout 30.4 Elementary Particles First we studied atoms Next, atoms had electrons and a nucleus The nucleus is composed of neutrons and protons What’s next? Elementary particle interactions The scattering of two electrons via a coulomb force This virtual photon is said to mediate the electromagnetic force. The virtual photon can never be detected because it only lasts for a vanishing small time. An simple example of a Feynman diagram Interactions continued Can have similar diagrams with other particles and other forces Strong force, weak force, gravity Basic idea of exchange of a virtual particle is the common theme. More examples of Feynman diagrams 30.5 The Fundamental Forces in Nature Strong Force Short range ~ 10-15 m (1 fermi) Responsible for binding of quarks into neutrons and protons Gluon Electromagnetic Force 10-2 as strong as strong force 1/r2 force law Binding of atoms and molecules Photon Weak force ~ 10-6 times as strong as the strong force Responsible for beta decay, very short range ~10-18 m W+, W- and Z0 bosons Gravitational Force 10-43 times as strong as the strong force Also 1/r2 force law Graviton 30.6 Positrons and Antiparticles Dirac proposed the positron to solve a negative energy problem (Dirac sea) The general implication is that for every particle there is an antiparticle (symmetry) Other antiparticles: antiproton, antineutrino Usually denoted with a bar over symbol Some particles are their own antiparticles photon, neutral pion: , 0 30.7 Mesons Part of an early theory to describe nuclear interactions Mass between a electron and a proton Flavors Charged meson: , ,mass 139.6 MeV/c2 Netral meson, 0 ,mass 135.0 MeV/c2 Lifetimes 2.6x10-8 s for , 8.3x10-17 s for 0 More Mesons Also have heavier mesons Kaons ~500 MeV/c2 Eta’s 548 and 958 MeV/c2 (note, mass of is greater than proton mass)