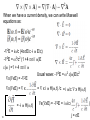* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Hall Effect, AC Conductivity and Thermal Conductivity
Electron configuration wikipedia , lookup
Atomic theory wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Matter wave wikipedia , lookup
Electron scattering wikipedia , lookup
Ferromagnetism wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
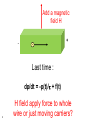
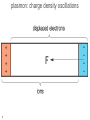
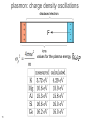
What happens to the current if we: 1. add a magnetic field, 2. have an oscillating E field (e.g. light), 3. have a thermal gradient H Add a magnetic field H - - + Last time : dp/dt = -p(t)/ + f(t) 2 H field apply force to whole wire or just moving carriers? Hall Effect In a current carrying wire when in a perpendicular magnetic field, the current should be drawn to one side of the wire. As a result, the resistance will increase and a transverse voltage develops. H Lorentz force = -ev/c x H ------------------ ++++++++++++++++ - + Similar approach: dp/dt = -p(t)/ + f(t) If a current flows (of velocity vD) in the positive x direction and a uniform magnetic field is applied in the positive zdirection, use the Lorentz force to determine the magnitude and direction of the resulting Hall field, first in terms of velocity, but then current density. Group • • • • Ey= vxHy/c = jxHy/nec Hall coefficient RH= Ey/jxH = 1/nec Lorentz force = -ev/c x H R is very small for metals as n is very large. Useful for calculating carrier density and type Hz -----------------++++++++++++++++ - x y The Hall coefficient Ohm’s law contains e2 But for RH the sign of e is important. A hole is the lack of an electron. It has the opposite charge so +e. Application: For a 100-m thick Cu film, in a 1.0 T magnetic field and through which I = 0.5 A is passing, the Hall voltage is 0.737 V. H H nec Not yet prepared to discuss other quantum versions of the Hall effect With strong magnetic fields: The integer quantum Hall effect is observed in 2D electron systems at low temperature, in which the Hall conductance undergoes quantum Hall transitions to take on quantized values from D.C. Tsui, RMP (1999) and from H.L. Stormer, RMP (1999) The fractional quantum Hall effect: Hall conductance of 2D electrons shows precisely quantized plateaus at fractional values of e2/h What happens to the electrons if we: have an oscillating E field (e.g. light) plasmon: charge density oscillations 9 Longitudinal Plasma Oscillations Displacement of the entire electron gas a distance d with respect to the positive ion background. This creates surface charges s = nde & thus an electric field E = 4nde. Equation of Motion: F = ma = -eE d Nm 2 NeE Ne(4nde) t 2 Oscillations at the d 2 Plasma Frequency d 0 p 2 t 2 4ne 2 p 2 m plasmon: charge density oscillations values for the plasma energy 11 Why are metals shiny? Drude’s theory gives an explanation of why metals do not transmit light and rather reflect it. Continuum limit: Where the wavelength is bigger than the spacing between atoms. Otherwise diffraction effects dominate. (Future topic) AC Electrical Conductivity of a Metal Newton’s 2nd Law Equation of dp( , t ) p( , t ) eE( , t ) Motion for the momentum of one dt electron in a time dependent E( , t ) E( )e it electric field. Look for a steady p( , t ) p( )e it state solution of the form: eE( ) p( ) j( ) s ( )E( ) AC conductivity DC conductivity s0 s ( ) 1 i ne 2 s0 1 / i nep( ) ( ne 2 / m )E( ) j( ) m 1 / i m Works great for the continuum limit when can treat the force on each electron the same. When we have a current density, we can write Maxwell equations as: -2E = i/c (4sE/c -i E/c) -2E = 2/c2 (1 +4 si / )E ( ) =1 + 4 si / Usual wave: -2E = 2 ()E/c2 x(xE) = -2E x(xE) = x = -i H(,t) 14 = x i H(,t) /c = i /c x H(,t) x(xE) = -2E = i /c ( j = sE ) From continuum limit s0 ne s ( ) ,s 0 1 i m From Maxwell’s equations 2 ( ) =1 + 4 si / Plugging s into : ( ) =1 + 4 s0i / (1-i ) =1 + 4 s0i / ( -i 2) Plugging in s0: ( ) =1 + 4 ne2i / m( -i 2) For high frequencies 1can ignore first term in denominator Ignoring: ( ) =1 - 4 ne2/m2 p is known as the plasma frequency 15 What is a plasma? Application to Propagation of Electromagnetic Radiation in a Metal E exp( it iK r ) The electromagnetic wave equation in a nonmagnetic isotropic medium. 2 2 2 ( , K ) / c K Look for a solution with the dispersion 2E = 2 ()E/c2 - relation for electromagnetic waves (1) real & > 0 → for real, K is real & the transverse electromagnetic wave propagates with the phase velocity vph= c/1/2 E/t= -i E(,t) 2E/t2= 2 E(,t) 2E = -2 ()E/c2 =2 E Transverse optical modes in a plasma Dispersion relation for (, K ) 2 c2 K 2 electromagnetic waves 2 p 2 c2 K 2 (1) real & positive, no damping (2) (p>) real & < 0 → for real, K ( ) is imaginary & the wave is damped with a characteristic length 1/|K| (Why metals are shiny) E e Kr (3) complex → for real, K is complex & the wave is damped in space (4) = 0 longitudinally polarized waves are possible / p (2) E&M waves are totally reflected from the medium when is negative (1) E&M waves propagate with no damping when is positive & real Ultraviolet Transparency of Metals Plasma Frequency p & Free Space Wavelength lp = 2c/p Range Metals n, cm-3 1022 p, Hz 5.7×1015 lp, cm 3.3×10-5 spectral range UV Semiconductors 1018 5.7×1013 3.3×10-3 IF Ionosphere 1010 5.7×109 33 radio The Electron Gas is Transparent when > p i.e. l < lp p 2 4ne m 2 The reflection of light from a metal is similar to the reflection of radio waves from the Ionosphere! Plasma Frequency Ionosphere Semiconductors Metals reflects transparent for metal visible UV ionosphere radio visible What happens when you heat a metal? What do we know from basic thermo 19 th (0 law)? Drude’s Best Success: Explanation of the Wiedemann-Franz law for metals (1853) Thermal conductivity Temperature Electrical conductivity • • • 20 Wiedemann and Franz observed that the ratio of thermal and electrical conductivity for ALL METALS is constant at a given temperature (for room temperature and above). Later it was found by L. Lorenz that this constant is proportional to the temperature. Let’s try to reproduce the linear behavior and to calculate the slope Thermal conductivity A material's ability to conduct heat. Electric current density (je = I/A) Heat current density Fourier's Law for heat conduction. E Thermal current density jt sec area jt vn = Energy per particle v = velocity n = N/V 2l Thermal conductivity Heat current density jt vn Heat Current Density jtot through the plane: jtot = jright - jleft Heat energy per particle passing through the plane started an average of “l” away. About half the particles are moving right, and about half to the left. x Thermal conductivity Heat current density x Limit as l gets small: Thermal conductivity x v v v Thermal conductivity Heat current density x T T x v 2 vx 1 2 jt v cv T jt T 3 2 vy 2 vz 2 3 vx 2 1 2 v cv 3 How does it depend on temperature? Thermal conductivity 1/3 cv = 2 v 2 ne /m 1/3 cvmv2 = 2 ne Drude applied ideal gas law ½ mv2 = 3/2 kBT = cvkBT ne2 The book jumps through claiming a value for cv Classical Theory of Heat Capacity When the solid is heated, the atoms vibrate around their sites like harmonic oscillators. The average energy for a 1D oscillator is ½ kT. Therefore, the average energy per atom, regarded as a 3D oscillator, is 3/2 kBT, and consequently the total energy is 3/2 nkBT where n is the conduction electron density and kB is Boltzmann constant. Differentiation w.r.t temperature gives heat capacity 3/2 n kB = 3/2 n kB2T ne2 = 3kB2T / 2e2 Thermal conductivity optimization To maximize thermal conductivity, there are several options: • Provide as many conduction electrons as possible • • Make crystalline instead of amorphous • • irregular atomic positions in amorphous materials scatter phonons and diminish thermal conductivity Remove grain boundaries • • free electrons conduct heat more efficiently than phonons. gb’s scatter electrons and phonons that carry heat Remove pores (air is a terrible conductor of heat) Many open questions: • • Why does the Drude model work so relatively well when many of its assumptions seem so wrong? In particular, the electrons don’t seem to be scattered by each other. Why? Why do the electrons not seem to contribute to the heat capacity? From Wikipedia: "The simple classical Drude model provides a very good explanation of DC and AC conductivity in metals, the Hall effect, and thermal conductivity (due to electrons) in metals. The model also explains the Wiedemann-Franz law of 1853. "However, the Drude model greatly overestimates the electronic heat capacities of metals. In reality, metals and insulators have roughly the same heat capacity at room temperature.“ It also does not explain the positive charge carriers from the Hall effect. Failures of the Drude model: electrical conductivity of an alloy • • 30 The resistivity of an alloy should be between those of its components, or at least similar to them. It can be much higher than that of either component. FYI: measurable quantity – Hall resistance H RH H Vy H More detail about H Ix n2 D ec Hall resistance E j j sE in the presence of magnetic field the resistivity and conductivity becomes tensors xx xy Ex xx xy jx for 2D: j E yy yy y yx y yx s 0 Ex cj y jx s 0 E y cjx j y c Ex jx jy s0 s0 c 1 Ey jx jy s0 s0 1 c s 0 1 s0 c s 0 1 s 0 c s 0 jx Ex 1 s 0 E j s 1 s 0 0 y y c 1 m xx 2 s 0 ne c H xy s 0 nec for 3D systems n2 D nLz for 2D systems n2D=n jx s xx s xy Ex j s E s yy y y yx s xx s xy xx s s yx s yy yx xy yy s0 s xx s yy 1 (c ) 2 s 0c s xy s yx 1 (c ) 2 xx s xx 2 xx xy 2 xy s xy 2 xx xy 2 1