* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ACD CPR - Rackcdn.com
Survey
Document related concepts
Transcript
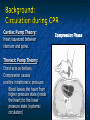
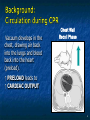
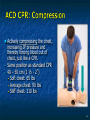
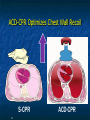
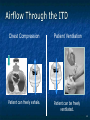
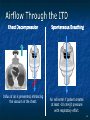
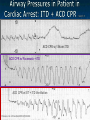
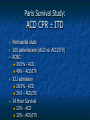
The Devil is in the Details R. J. Frascone, MD, FACEP 1 Medical Director EMS Regions Hospital EMS, St. Paul, MN Professor of Emergency Med University of Minnesota These trials were studying two different things, in two very different ways The RESQ Trial studied the combination of ACD/ITD CPR vs S-CPR ROC PRIMED studied ITD CPR vs S-CPR But, The RESQ Trial looked at only ACD/ITD vs S-CPR ROC PRIMED looked at two different things: Early vs late defibrillation and ITD CPR vs S-CPR and they did it with a multifactoral approach Both trials were complex, but PRIMED was extraordinarily complex First A Review (sorry) Standard CPR Cardiac Pump Theory: Heart squeezed between sternum and spine. Thoracic Pump Theory: Chest acts as bellows. Compression causes positive intrathoracic pressure: Blood leaves the heart from higher pressure state (inside the heart) to the lower pressure state (systemic circulation) Compression Phase 7 Vacuum develops in the chest, drawing air back into the lungs and blood back into the heart (preload). Chest Wall Recoil Phase ↑ PRELOAD leads to ↑ CARDIAC OUTPUT 8 10 Inefficiency #1 Filling of the heart (preload) is dependent upon the chest wall’s ability to recoil during decompression phase. Chest wall recoil may be compromised by: A stiff chest Broken ribs Just doing it wrong 11 Inefficiency #2 Air rushes in through an open airway and wipes out the vacuum we’re relying on to fill the heart. Heart stops filling as soon as vacuum is equalized. 13 ACD CPR Metronome Force Gauge Suction Cup Handle 15 Actively compressing the chest, increasing IP pressure and thereby forcing blood out of chest, just like s-CPR. Same position as standard CPR 40 – 50 cm (1 ½ - 2”) Soft chest: 65 lbs Average chest: 90 lbs Stiff chest: 110 lbs 16 But, unlike S-CPR it actively decompresses the chest, decreasing IP pressure, thereby drawing blood into the chest. Typically 15-20 lbs 17 ACD-CPR Optimizes Chest Wall Recoil S-CPR 18 ACD-CPR Does it Work? Standard vs. ACD CPR: Human Study Survival Plaisance, P, Lurie, KG, et al. NEJM. 1999 Aug;341(8):569-575. Standard or ACD CPR during ACLS only 45 40 1.5 35 1.5 1.4 1.9 25 standard CPR 20 ACD CPR 15 (n=377) (n=373) 2.0 10 3.2* 5 2.5* Iy r 7d ay s 24 hr Di sc ha rg e* * IC U ad mi ss io n 1h r 0 RO SC survivors (%) 30 Odds ratios shown above bars * Statistically significant difference ** Discharge without neurologic impairment Standard vs. ACD CPR: Human Study Survival Plaisance, P, Lurie, KG, et al. NEJM. 1999 Aug;341(8):569-575. Standard or ACD CPR during BLS and ACLS 55 50 45 35 30 standard CPR 25 ACD CPR 20 (n=99) (n=120) 15 10 5 Iy r Di sc ha rg e* hr 24 ad m iss io n N- IC U 1 hr 0 RO SC survivors (%) 40 * Discharge without neurologic impairment ACD CPR The Problem is: Air rushes in through an open airway and wipes out the vacuum we’re relying on to fill the heart. Heart stops filling as soon as vacuum is equalized. The Solution Enter the Impedence Threshold Device (ITD) Chest Compression Patient Ventilation Patient can freely exhale. Patient can be freely ventilated. 25 Chest Decompression Influx of air is prevented, enhancing the vacuum in the chest. Spontaneous Breathing Air will enter if patient creates at least -10 cmH2O pressure with respiratory effort. 26 Greater vacuum (negative pressure) in the chest during chest wall recoil phase 27 ACD CPR w/ Sham ITD ACD CPR w/ Facemask + ITD ACD CPR w/ ET + ITD Ventilation Plaisance et al. Crit Care Med 2005;33(5):990-994 28 mmHg Improved Blood Pressure P<0.05 for differences between S-CPR & S-CPR + ITD, and ACD-CPR & ACD-CPR + ITD 29 Pirrallo et al. Resuscitation 2005;(66):13-20 and Plaisance et al. Circulation 2000;(101):989-994. Many other trials both in animals and human that prove the effectiveness of the ITD alone or in combination with ACD Putting it all together ACD/ITD CPR in humans Human Study ACD CPR +/- Valve: Plaisance, P, Lurie, KG, Payen, D. Circ. 2000;101:989-994 End-Tidal CO2 22 With Valve Without Valve 20 22 n=8 16 n=11 14 n=7 n=10 End-Tidal CO2 n=10 18 End-Tidal CO2 24 n=9 20 18 16 14 12 10 8 6 4 12 0 n=10 n=11 Time (min) n=10 10 n=10 8 n=11 n=8 n=10 6 4 0 5 10 15 5 10 15 20 25 20 25 30 Duration of CPR (minutes) ACD CPR +/- Valve: Human Study Diastolic Arterial Pressure 60 Plaisance, P, Lurie, KG, Payen, D. Circ. 2000;101:989-994 n=9 Diastolic Arterial Pressure (mm Hg) n=11 n=8 n=7 50 With Valve Without Valve 40 n=10 n=10 n=10 60 50 40 30 15 20 Time (min) 30 20 15 70 10 n=10 n=8 10 Diastolic Arterial Pressure (mm Hg) n=10 20 25 30 Duration of CPR (minutes) 25 ACD CPR +/- Valve: Human Study Coronary Perfusion Pressure Coronary Perfusion Pressure (mmHg) 50 n=10 45 n=9 n=11 n=8 40 n=7 With Valve 35 Without Valve Coronary Perfusion Pressure (mmHg) Plaisance, P, Lurie, KG, Payen, D. Circ. 2000;101:989-994 60 50 40 30 20 10 10 15 20 Time (min) 30 n=10 n=10 n=10 n=10 25 n=8 20 15 10 15 20 25 30 Duration of CPR (minutes) 25 Paris Survival Study: ACD CPR ITD Prehospital study 200 patients/arm (ACD vs. ACD/ITV) ROSC: ICU admission 38.5% - ACD 48% - ACD/ITV 28.5% - ACD 39.5 – ACD/ITV 24 Hour Survival 22% - ACD 32% - ACD/ITV The ResQ Trial Tom P. Aufderheide, MD; Ralph J. Frascone, MD; Marvin A. Wayne, MD; Brian D. Mahoney, MD; Robert A. Swor, DO; Robert M. Domeier, MD; Michael L. Olinger, MD; Richard G. Holcomb, PhD; David E. Tupper, PhD; Demetris Yannopoulos, MD; Keith G. Lurie, MD 37 S-CPR (Control) ITD + ACD-CPR (Intervention) 39 Survival to hospital discharge with favorable neurologic function (measured with a modified Rankin Scale [mRS] ≤ 3), is higher in patients receiving an ITD + ACD-CPR compared to patients receiving Standard CPR (S-CPR). 40 Prospective, randomized, controlled clinical trial with data analyzed on intent to treat basis Seven US sites (population base: 2.3 million): 46 EMS agencies 4950 EMS providers 25 IRBs Patients assigned, based upon weekly block randomization, to control or intervention group Study period: February 2005 – July 2010 All study personnel blinded to aggregate data 41 Results Survival to Hospital Discharge with Favorable Neurologic Outcome * *53% improvement P = 0.019 OR 1.58 CI (1.07, 2.36) 43 Age at Time of Arrest (years) 44 Survival to Hospital Discharge with Favorable Neurologic Outcome Survival to Hospital Discharge with Favorable Neurologic Outcome P=1.00 for differences based on gender Odds ratio for effect of intervention based on gender: 1.60 95% CI (1.10, 2.33) 45 Survival to Hospital Discharge with Favorable Neurologic Outcome Cumulative Enrollment 2006 2007 2008 2009 Control 5 172 387 713 Intervention 6 168 395 703 11 340 782 1416 Total Control (N = 813) One-Year Survival Emotional: Beck Depression Inventory (BDI) Intervention (N = 840) P-value 48 (5.9%) 74 (8.8%) 0.030 5.2 ± 6.3 5.5 ± 5.9 0.862 1.4 ± 3.1 2.2 ± 5.7 0.358 92.9 ± 12.0 94.5 ± 4.5 0.473 (Score range: 0 – 63) Functional: Disability Rating Score (DRS) (Score range: 0 – 29) Cognitive: Cognitive Abilities Screening Instrument (CASI) Score range: (0 – 100) 47 Compared to standard CPR, ITD + ACD-CPR resulted in significantly increased survival to hospital discharge with favorable neurological function (53%). One year after OOHCA, survival rates with similar neurologic function were also significantly higher in the intervention group (49%). 48 Aufderheide et al. A trial of an impedance threshold device in out-of-hospital cardiac arrest. NEJM 2011365;798-806. 49 Purpose To determine if use of an active (versus sham) ITD during standard CPR (no ACD used) would improve rates of hospital discharge with functional neurological survival in adult (modified Rankin Scale [mRS] score ≤3), non-traumatic, out of hospital cardiac arrests Description/Methods 10 sites in US and Canada Prospective, randomized, blinded Subjects: adults with arrest from presumed cardiac etiology 2 x 2 multivariate study design Analyze Early (30 secs CPR) vs Analyze Later (3 min CPR) Stiell et al. NEJM 2011 Sham vs Active ITD Aufderheide et al. NEJM 2011 Impact of immediate CPR feedback utilizing QCPR device @ three sites Hostler et al. BJM 2011 Results Results Overall results in sham vs active ITD were similar (≈6%) November 2, 2009, NIH announced study terminated early (at the 2/3 enrollment point) as it was not going to be possible to detect any overall significant difference between either of the study groups (AnE vs AnL, or sham vs active ITD) even if study continued to 14,000 patients (stopped because of futility) No safety concerns with ITD Conclusion Compared with standard CPR, use of the ITD did not significantly improve functional survival from out-ofhospital cardiac arrest. When implemented under similar conditions, routine use of the ITD is not supported. What are the Problems with ROC? The Devil is in the Details Protocols Three different BLS protocols ALS protocols per site medical director Various ROC Study Protocols BLS CPR Method ITD Study1 30:2 compression to ventilation ratio Sham vs Active ITD Continuous chest compressions with asynchronous ventilations @ 10/min 1Aufderheide 57 et al. NEJM 2011 AE vs AL Study2 30 secs vs 180 secs of CPR before analyze and shock Did not participate 2Stiell et al. NEJM 2011 QCPR Study3 Sites Participating Did not participate Milwaukee, WI Dallas, TX San Diego, CA Portland, OR Birmingham, AL Ottawa, CA Toronto, CA Feedback ON vs Feedback OFF Pittsburgh, PA Thunder Bay, ON Did not participate Vancouver, CA Feedback ON vs Feedback OFF Seattle (King County), WA 3Hostler et al. BMJ 2011 Study Protocol Respond to scene and determine pulselessness. Perform 1 – 4 simultaneously: 1.Review enrollment criteria for AE vs AL study a) If eligible and in V-Fib, perform either 30 secs or 3 min of CPR prior to analyzing and shocking if indicated. b) If eligible and in asystole or PEA, or not eligible, perform conventional resuscitation 2.Review enrollment criteria for QCPR study a) If eligible, place QCPR device; then, based upon cluster randomization, audible and visual feedback either will or will not be given to rescuers. b) If not eligible, do not place QCPR device and perform conventional resuscitation. 3.Review enrollment criteria for ITD study a) If eligible, select ITD in serialized order and place on patient. b) If not eligible, perform conventional resuscitation. 4.Perform other conventional activities of resuscitation (e.g. airway management, IV/IO, administer medications, defibrillate) ROC ITD Placement Intervals First/BLS Response ALS Response Dispatch to first unit arrival 5.8 9.0 Arrival of rig carrying ITD to application 4.0 4.0 Time from 911 call to dispatch of EMS (estimated) 1.0 1.0 10.8 minutes 14.0 minutes Time Intervals (minutes) Estimated mean ITD placement interval 59 Device Placement Intervals ROC PRIMED Study First/BLS Response 5.8 ALS Response 9.0 Arrival of rig carrying ITD to application 4.0 4.0 911 call to dispatch of EMS (estimated) 1.0 1.0 Estimated mean ITD placement interval 10.8 min 14.0 min Median Time Intervals (minutes) Dispatch to first unit arrival ResQTrial Study Mean Time Intervals (minutes) 911 call to EMS CPR start time Time it took EMS to place devices once CPR begun 911 call to device placement 60 EMS Response 6.7 0.4 (24 secs) 7.1 min Problems Complicated Analyze Early vs Analyze Later & QCPR protocols, multivariate design caused multiple problems: ITD placement was very delayed (up to 14 minutes) Almost 40% of cases did not have ITD placed within the planned time interval (under 5 minutes) All the patients who survived in under approximately 4 minutes were not eligible for the ITD Essentially all cases of early use were on asystolic patients Treatment protocols were inconsistent ResQTrial: Impact of Time to Device Placement on Survival Survival to Hospital Discharge with Favorable Neurological Outcome (%) Average Time of Device Placement in ResQTrial (7.1 min) Time from 911 Call to Randomized CPR Method (min) 62 ROC PRIMED All the patients who survived in under approximately 4 minutes were not eligible for the ITD. The Bottom Line Two Very Different Studies ResQ Trial studied ACD/ITD Combination ROC PRIMED studied ITD Alone In Addition… Early survivors were excluded from getting the ITD (under 4 minutes) ITD was used early on probable, non-survivors (asystole) ITD way to late for the device to be successful 66