* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download DIAGNOSIS OF SWINE FLU
2015–16 Zika virus epidemic wikipedia , lookup
Public health genomics wikipedia , lookup
Transmission (medicine) wikipedia , lookup
Human mortality from H5N1 wikipedia , lookup
Herpes simplex research wikipedia , lookup
Infection control wikipedia , lookup
Influenza A virus subtype H5N1 wikipedia , lookup
Marburg virus disease wikipedia , lookup
Viral phylodynamics wikipedia , lookup
Avian influenza wikipedia , lookup
Henipavirus wikipedia , lookup
Canine parvovirus wikipedia , lookup
Transmission and infection of H5N1 wikipedia , lookup
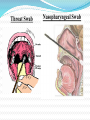
Influenza A H1N1 (Swine flu) DIAGNOSIS OF SWINE FLU For diagnosis of swine influenza A infection, respiratory specimen would generally need to be collected within the first 4 to 5 days of illness. However, some persons, especially children, may shed virus for 10 days or longer. The following clinical samples need to be collected preferably within 72 hours of illness and sent to the laboratory with in 24 hours of collection. Nasopharyngeal Wash/Aspirate. Nasopharyngeal (NP) swab/ Oropharyngeal (OP) swab/Throat swab (TS) Swab specimens should be collected only on swabs with a synthetic tip (such as polyester or Dacron) and aluminium or plastic shaft. Swabs with cotton and wooden shafts are not recommended. Throat Swab Nasopharyngeal Swab • Storage of Samples: all samples should be kept at 2- 8C until they can be placed at-70C. • Transportation of Samples: Clinical samples should be transported on dry ice in triple packaging. All samples should be labeled clearly and include patient’s complete information and should be sent to NIV, Pune or NICD, Delhi within 24 hours for further investigations. Available Laboratory tests Rapid Antigen Tests RT-PCR Virus isolation Virus Genome Sequencing Four-fold rise in swine influenza A (H1N1) virus specific neutralizing antibodies. Biosafety for Laboratory Workers Respiratory protection – fit-tested N95 respirator or higher level of protection. Shoe covers Closed-front gown Double gloves Eye protection (goggles or face shields) Appropriate disinfectants 70% Ethanol 5% Lysol 10% Bleach Antiviral Treatment Oseltamivir (TAMIFLU) is the recommended drug both for prophylaxis and treatment. Other drug: Zanamivir (RELENZA) Both are nuraminidase inhibitors. Oseltamivir Body weight Treatment Prophylaxis < 15 kg 30mg twice a 30mg once a day for 5 days day for 10 days 15-23 kg 45 mg twice a 45 mg once a day for 5 days day for 10 days 24 - < 40 kg 60 mg twice a 60 mg once a day for 5 days day for 10 days > 40 kg 75 mg twice a 75 mg once a day day for 5 days for 10 days Infants less than 1 year Age in months/ Dosage (Syrup Treatment 12mg/ml) < 3 months 3-5 months 6-11 months Prophylaxis 12 mg twice a Not recommended unless day for 5 days situation judged critical 20 mg twice a 20 mg once a day for 10 day for 5 days days after last exposure 25 mg twice a 25 mg once a day for 10 day for 5 days days after last exposure Antiviral Chemoprophylaxis All close contacts of suspected, probable and confirmed cases. Close contacts include household /social contacts, workplace or school contacts, fellow travelers etc. All health care personnel coming in contact with suspected, probable or confirmed cases. Oseltamivir and Zanamivir Oseltamivir and Zanamivir are inhibitors of the influenza viral neuraminidase enzyme, which is essential for release of the virus from infected cells. The enzyme cleaves terminal sialic acid residues and thus destroys the cellular receptors to which the viral hemagglutinin attaches. Both are sialicacid transitionstate analogues and are highly active and specific inhibitors of the neuraminidases of influenza A. Conti… Both act through competitive and reversible inhibition of the active site of influenza A viral neuraminidases and have relatively little effect on mammalian cell enzymes. Oseltamivir phosphate is an ethyl ester prodrug that is converted to oseltamivir carboxylate by esterases in the liver. Orally administered oseltamivir has a bioavailability of >60% and a plasma half-life of 7–9 h. The drug is excreted unmetabolized, primarily by the kidneys. Conti… The toxicities most frequently encountered with orally administered oseltamivir are nausea, gastrointestinal discomfort, and (less commonly) vomiting. Gastrointestinal discomfort is usually transient and is less likely if the drug is administered with food. Recently, neuropsychiatric events (delirium, self-injury) have been reported in children who have been taking oseltamivir, primarily in Japan. Supportive therapy IV Fluids. Oxygen therapy/ ventilatory support. Antibiotics for secondary infection. Vasopressors for shock. Paracetamol or Ibuprofen Salicylate / aspirin is strictly contra-indicated in any influenza patient due to its potential to cause Reye’s syndrome. Vaccines Currently, most of the world's flu vaccines use an injection of "killed virus " The vaccine is administered by SC or IM route. A single inoculation (0.5ml for >3 yrs and 0.25 ml for <3 yrs) is given. Protective value 70-90%. Immunity lasts for only 6-12 months. Preparedness at National Level (GOI) Inter Ministerial Task Force monitoring the situation. Travel advisory issued to defer non-essential travel to the affected countries. Health screening of passengers from affected countries in 21 International airports. 191 doctors and 101 paramedics have been deployed to man 76 counters at the above airports. Conti… Guidelines issued on clinical management, infection control practices and Lab support; and intensified Surveillance in partnership with WHO. Stockpile of Tamiflu increased to 10 million doses. Stockpile of PPE increased to 1 million. IEC activities initiated in partnership with UNICEF. Laboratory Support 1. National Institute of Communicable Diseases, New 2. 3. 4. 5. 6. 7. 8. Delhi. National Institute of Virology, Pune. National Institute of Cholera & Other Enteric Diseases, Kolkata. Department of Microbiology, AIIMS. Enterovirus Research Centre, Mumbai. Vector Control and Research Centre, Pondicherry. Centre for Research in Medical Entomology, Madurai. Defence Research Development Establishment, Gwalior. Standard Operating Procedure for Entry Screening and Exit Screening The health desks would be set up before the immigration area. A space of 1sq m would be required for the desk and at least five metre in front of it for maintaining que. The desks would be separated by at least a meter. An approximate bench mark would be one desk per 1000 passenger. In addition there would be an examination room, a small space for storage of equipments and consumables and a space of 5m x 3m for isolating a suspect case. At the Health Desk Ask for any history of travel to affected area/country. The body temperature would be recorded. If already febrile, then duty Nurse would put the face mask on suspect ill traveler. He/she would be subjected to detail clinical examination. Query of the passenger, if any, would be entertained. Use alcoholic hand rub / sanitizer / soap and water for frequent hand wash. The District Level District Collector/ District Magistrate to assume over all coordination of the operations at the District level. Important functionaries in the district, particularly the CDHO, Superintendent of the Police, Chief Executive of the Panchayat Raj and Urban Local bodies will be members of the committee. Role of District Collector / Magistrate Hold meetings with District functionaries Develop district action plan. This would include: Details of RRT along with Contact numbers Number of RRTs that can be set up in the District. Lab linkages to be established. Listing of health facilities available in the District with details. Details human resource other than the existing work force. Conti… Police force available in the District. Availability of medicines and PPEs Availability of vehicles for transportation of RRTs, drugs etc. Legislative provisions required for ordering restricted movement, closure of schools, colleges, markets, cinemas and other places of public congregation. Test existing plan through Mock drill Conti… Co-ordinate with the concerned officers of the State Government and District Collectors of neighbouring districts and keep them appraised of the situation. Preparing a media plan and IEC materials. Keeping a watch on the news reports as well as daily briefing s of the MOHFW and if required scorching all rumours relating to the disease or its spread. Plan for maintaining essential services and continuity of operations. Assess budget requirements and identify the source. Preventive Measures Avoid close contact with people who are having respiratory illness. Sick persons should keep distance from others. Cover your mouth and nose with a tissue or handkerchief when coughing or sneezing. Washing your hands often with soap or alcohol based hand wash will help protect from germs. Persons who develop influenza-like-illness (ILI) should be strongly encouraged to selfisolate in their home for 7 days after the onset of illness or at least 24 hours after symptoms have resolved, whichever is longer. When the ill person is within 6 feet of others at home, the ill person should wear a face mask, if available or handkerchief or tissues. Get plenty of sleep, be physically active, manage your stress, drink plenty of fluids, and eat nutritious food. Precautions for School children Schools with a confirmed or a suspected case should be considered for closure. All school or childcare related gatherings should be cancelled and encourage parents and students to avoid congregating outside of the school. If no additional confirmed or suspected cases are identified among students (or school-based personnel) for a period of 7days, schools may consider reopening. Social Distancing Interventions Mass gatherings such as festivals, sporting, religious, political events need to be discouraged and cancelled. Funeral gatherings, in particular, needs to be discouraged. General public entry to airports and railway stations etc would be restricted. Public transportation may have to be restricted. Persons with underlying medical conditions who are at high risk for complications of influenza may wish to consider avoiding large gatherings. Home based Preparedness during pandemic phase Stockpile for at least 6 weeks the following: Non perishable food items – ready to eat food, cereals, pulses, sugar, salt, spices, oil, tea, coffee powder, powdered milk, etc. Potable water, cooking fuel. Emergency medical kits. Prescribed medications for chronic patients. Soap/Alcoholic handrubs, tissues. Batteries. Fundamentals of infection prevention strategies Administrative control are key components, including: implementation of Standard and Droplet Precautions; avoid crowding, promote distance between patients (≥ 1 m); patient triage for early detection, patient placement and reporting; organization of services; policies on rational use of available supplies; policies on patient procedures; strengthening of infection control infrastructure. Post-Peak Period During this period, pandemic disease levels in most countries with adequate surveillance will have dropped below peak observed levels. The post-peak period signifies that pandemic activity appears to be decreasing; however, it is uncertain if additional waves will occur and countries will need to be prepared for a second wave. Post-Pandemic Period In this period, influenza disease activity will have returned to levels normally seen for seasonal influenza. It is expected that the pandemic virus will behave as a seasonal influenza A virus. At this stage, it is important to maintain surveillance and update pandemic preparedness and response plans accordingly. An intensive phase of recovery and evaluation may be required. Steps of Hand Washing Personal Protective Equipments Debate over name In the Netherland, it was originally called "pig flu", but is now called "Mexican flu " South Korea and Israel briefly considered calling it the "Mexican virus" Taiwan suggested the names "H1N1 flu" or "new flu" The World Organization for Animal Health has proposed the name "North American influenza " The European Commission uses the term "novel flu virus". The WHO announced they would refer to the new influenza virus as influenza A (H1N1) or "Influenza A (H1N1) virus, human" THANK YOU Issues to be answered on Vaccine? “What happens if the virus mutates when the vaccine is ready? How much should be produced? How will it be distributed? Who should get it?"