* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Human Immunodeficiency Virus (HIV)
Adaptive immune system wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Globalization and disease wikipedia , lookup
Innate immune system wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
DNA vaccination wikipedia , lookup
Molecular mimicry wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
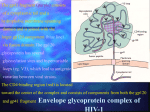
Human Immunodeficiency Virus (HIV) Terry Kotrla, MS, MT(ASCP)BB Fall 2005 Introduction • Etiologic agent of Acquired Immunodeficiency Syndrome (AIDS). • Discovered independently by Luc Montagnier of France and Robert Gallo of the US in 1983-84. • Former names of the virus include: – Human T cell lymphotrophic virus (HTLV-III) – Lymphadenopathy associated virus (LAV) – AIDS associated retrovirus (ARV) Introduction • HIV-2 discovered in 1986, antigenically distinct virus endemic in West Africa. • One million people infected in US, 30 million worldwide are infected. • Leading cause of death of men aged 2544 and 4th leading cause of death of women in this age group in the US. • http://www.cnn.com/2005/HEALTH/conditions/11/17/blacks.hiv.ap/ Characteristics of the virus • Icosahedral (20 sided), enveloped virus of the lentivirus subfamily of retroviruses. • Retroviruses transcribe RNA to DNA. • Two viral strands of RNA found in core surrounded by protein outer coat. – Outer envelope contains a lipid matrix within which specific viral glycoproteins are imbedded. – These knob-like structures responsible for binding to target cell. Characteristics of the virus HIV • The outer shell of the virus is known as the Viral enevlope. Embedded in the viral envelope is a complex protein known as env which consists of an outer protruding cap glycoprotein (gp) 120, and a stem gp14. Within the viral envelope is an HIV protein called p17(matrix), and within this is the viral core or capsid, which is made of another viral protein p24(core antigen). Structural Genes • Three main structural genes: – Group Specific Antigen (Gag) – Envelope (Env) – Polymerase (Pol) Group Specific Antigen (Gag) • Located in nucelocapsid of virus. • Icosahedryl capsid surrounds the internal nucleic acids made up of p24 andp15. • p17 lies between protein core and envelope and is embedded in the internal portion of the envelope. • Two additional p55 products, p7 and p9, are nucleic acid binding proteins closely associated with the RNA. Envelope (Env) • Envelope (Env) gene codes for envelope proteins gp160, gp120 and gp41. – These polyproteins will eventually be cleaved by proteases to become HIV envelope glycoproteins gp120 and gp41. – gp160 cleaved to form gp120 and gp41. – gp120 forms the 72 knobs which protrude from outer envelope. – gp41 is a transmembrane glycoprotein antigen that spans the inner and outer membranes and attaches to gp120. – gp120 and gp41 both involved with fusion and attachment of HIV to CD4 antigen on host cells. Polymerase (Pol) • Polymerase (Pol) codes for p66 and p51 subunits of reverse transcriptase and p31 an endonuclease. – Located in the core, close to nucleic acids. – Responsible for conversion of viral RNA into DNA, integration of DNA into host cell DNA and cleavage of protein precursors. Viral Replication • First step, HIV attaches to susceptible host cell. – Site of attachment is the CD4 antigen found on a variety of cells • • • • • • helper T cells macrophages monocytes B cells microglial brain cells intestinal cells – T cells infected later on. Early Phase HIV Infection • In early phase HIV infection, initial viruses are M-tropic. Their envelope glycoprotein gp120 is able to bind to CD4 molecules and chemokine receptors called CCR5 found on macrophages http://www.cat.cc.md.us/courses/bio141/lecguide/unit2/viruses/hivad.html • In late phase HIV infection, most of the viruses are T-tropic, having gp120 capable of binding to CD4 and CXCR4 found on T4lymphocytes. Life Cycle • • • • • • • • • • • (a) HIV (red) attaches to two cell-surface receptors (the CD4 antigen and a specific chemokine receptor). (b) The virus and cell membrane fuse, and the virion core enters the cell. (c) The viral RNA and core proteins are released from the virion core and are then actively transported to the nucleus. (d) The viral RNA genome is converted into doublestranded DNA through an enzyme unique to viruses, reverse transcriptase (red dot). (e) The double-stranded viral DNA moves into the cell nucleus. (f) Using a unique viral enzyme called integrase, the viral DNA is integrated into the cellular DNA. (g) Viral RNA is synthesized by the cellular enzyme RNA polymerase II using integrated viral DNA as a template. Two types of RNA transcripts shorter spliced RNA (h) and full-length genomic RNA (j) are produced. (h) Shorter spliced RNAs are transported to the cytoplasm and used for the production of several viral proteins that are then modified in the Golgi apparatus of the cell (i). (j) Full-length genomic RNAs are transported to the cytoplasm (k). (l) New virion is assembled and then buds off. (m) Mature virus is released. Viral Replication • The gp120 protein on virus binds specifically to CD4 receptor on host cell with high affinity. • Gp41 causes fusion of the virus to the cell membrane. – After fusion virus particle enters cell. – Viral genome exposed by uncoating particle. Viral Replication • Reverse transcriptase produces viral DNA from RNA. – Becomes a provirus which integrates into host DNA. – Period of latency occurs. • http://www.cat.cc.md.us/courses/bio141/lecguide/unit2/viruses/hivdsdna.html Viral Replication • After a period of latency lasting up to 10 years viral replication is triggered and occurs at high rate. • CD4 cell may be destroyed in the process, body attempts to replace lost CD4 cells, but over the course of many years body is unable to keep the count at a safe level. • Destruction of large numbers of CD4 cause symptoms of HIV to appear with increased susceptibility to opportunistic infections, disease and malignancy. HIV (arrows) Infecting a T-lymphocyte Viral Replication • Methods of transmission: – Sexual transmission, presence of STD increases likelihood of transmission. – Exposure to infected blood or blood products. – Use of contaminated clotting factors by hemophiliacs. – Sharing contaminated needles (IV drug users). – Transplantation of infected tissues or organs. – Mother to fetus, perinatal transmission variable, dependent on viral load and mother’s CD 4 count. Transmission Primary HIV Syndrome • Mononucleosis-like, cold or flu-like symptoms may occur 6 to 12 weeks after infection. – – – – – – – – – lymphadenopathy fever rash headache Fatigue diarrhea sore throat neurologic manifestations. no symptoms may be present Primary HIV Syndrome • Symptoms are relatively nonspecific. • HIV antibody test often negative but becomes positive within 3 to 6 months, this process is known as seroconversion. • Large amount of HIV in the peripheral blood. • Primary HIV can be diagnosed using viral load titer assay or other tests. • Primary HIV syndrome resolves itself and HIV infected person remains asymptomatic for a prolonged period of time, often years. Clinical Latency Period • HIV continues to reproduce, CD4 count gradually declines from its normal value of 5001200. • Once CD4 count drops below 500, HIV infected person at risk for opportunistic infections. • The following diseases are predictive of the progression to AIDS: – – – – persistent herpes-zoster infection (shingles) oral candidiasis (thrush) oral hairy leukoplakia Kaposi’s sarcoma (KS) Oral Candidiasis (thrush) Oral Hairy Leukoplakia • Being that HIV reduces immunologic activity, the intraoral environment is a prime target for chronic secondary infections and inflammatory processes, including OHL, which is due to the Epstein-Barr virus under immunosuppressed conditions Kaposi’s sarcoma (KS) • Kaposi’s sarcoma (shown) is a rare cancer of the blood vessels that is associated with HIV. It manifests as bluish-red oval-shaped patches that may eventually become thickened. Lesions may appear singly or in clusters. AIDS • CD4 count drops below 200 person is considered to have advanced HIV disease • If preventative medications not started the HIV infected person is now at risk for: – Pneumocystis carinii pneumonia (PCP) – cryptococcal meningitis – toxoplasmosis • If CD4 count drops below 50: – – – – – Mycobacterium avium Cytomegalovirus infections lymphoma dementia Most deaths occur with CD4 counts below 50. Other Opportunistic Infections • Respiratory system – – – • Gastro-intestinal system – – – – – • Cryptosporidiosis Candida Cytomegolavirus (CMV) Isosporiasis Kaposi's Sarcoma Central/peripheral Nervous system – – – – – – • Pneumocystis Carinii Pneumonia (PCP) Tuberculosis (TB) Kaposi's Sarcoma (KS) Cytomegolavirus Toxoplasmosis Cryptococcosis Non Hodgkin's lymphoma Varicella Zoster Herpes simplex Skin – – – Herpes simple Kaposi's sarcoma Varicella Zoster Infants with HIV • • • • • • • Failure to thrive Persistent oral candidiasis Hepatosplenomegaly Lymphadenopathy Recurrent diarrhea Recurrent bacterial infections Abnormal neurologic findings. Immunologic Manifestations • Early stage slight depression of CD4 count, few symptoms, temporary. • Window of up to 6 weeks before antibody is detected, by 6 months 95% positive. • During window p24 antigen present, acute viremia and antigenemia. Immunologic Manifestations • Antibodies produced to all major antigens. – First antibodies detected produced against gag proteins p24 and p55. – Followed by antibody to p51, p120 and gp41 – As disease progresses antibody levels decrease. Immunologic Manifestations • Immune abnormalities associated with increased viral replication. – Decrease in CD4 cells due to virus budding from cells, fusion of uninfected cells with virally infected cells and apoptosis. – B cells have decreased response to antigens possibly due to blockage of T cell/B cell interaction by binding of viral proteins to CD4 site. – CD8 cells initially increase and may remain elevated. – As HIV infection progresses, CD4 T cells drop resulting in immunosuppression and susceptibility of patient to opportunistic infections. – Death comes due to immuno-incompetence. Immunologic Manifestations • Immune abnormalities associated with increased viral replication. – Decrease in CD4 cells due to virus budding from cells, fusion of uninfected cells with virally infected cells and apoptosis. – B cells have decreased response to antigens possibly due to blockage of T cell/B cell interaction by binding of viral proteins to CD4 site. – CD8 cells initially increase and may remain elevated. – As HIV infection progresses, CD4 T cells drop resulting in immunosuppression and susceptibility of patient to opportunistic infections. – Death comes due to immuno-incompetence. The Move Toward Lower Pill Burdens Regimen Dosing 1996 Zerit/Epivir/Crixivan 10 pills, Q8H 1998 Retrovir/Epivir/Sustiva 5 pills, BID 2002 Combivir (AZT/3TC)/EFV 3 pills, BID 2003 Viread/ Emtriva/Sustiva 3 pills, QD 2004 Truvada/Sustiva 2 pills, QD Daily pill burden Sustiva + Truvada Treatment • Sustiva + Truvada (FTC + tenofovor) is one of the most popular and effective starting HIV regimens. • Many patients will have dream/sleep/central nervous system effects particularly in the first month (due to the Sustiva). • Upset stomach/bloating/gas/loose stools is also fairly common during the first month and for most patients is fairly mild. • HIV levels in the blood will often drop by > 99% in the first month and the CD4 count (marker of immune system function) will often increase providing protection against AIDS related diseases within weeks/months of starting the medication. Truvada • Truvada is made up of HIV drugs from a class called nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), also known as “nukes.” • The NRTIs block reverse transcriptase, a protein that HIV needs to make more copies of itself. This may slow down HIV disease ‘typical’ primary HIV-1 infection symptoms symptoms HIV proviral DNA HIV antibodies ‘window’ period HIV viral load HIV-1 p24 antigen 0 1 1° infection 2 3 4 5 6 / 2 weeks 4 6 years Time following infection 8 10 Laboratory Diagnosis of HIV Infection • Methods utilized to detect: – Antibody – Antigen – Viral nucleic acid – Virus in culture ELISA Testing • First serological test developed to detect HIV infection. – Easy to perform. – Easily adapted to batch testing. – Highly sensitive and specific. • Antibodies detected in ELISA include those directed against: p24, gp120, gp160 and gp41, detected first in infection and appear in most individuals ELISA Testing • ELISA tests useful for: – Screening blood products. – Diagnosing and monitoring patients. – Determining prevalence of infection. – Research investigations. ELISA Testing • Different types of ELISA techniques used: – indirect – competitive – sandwich • ELISAs are for screening only, false positives do occur and may be due to AI disease, alcoholism, syphilis, and immunoproliferative diseases. ELISA Sandwich Other Screening Tests • Agglutination tests using latex particles, gelatin particles or microbeads are coated with HIV antigen and will agglutinate in the presence of antibody. • Dot-Blot Testing utilizes paper or nitrocellulose impregnated with antigen, patient serum is filtered through, and anti-antibody is added with enzyme label, color change is positive. – A rapid, cost-effective and may become an alternative to standard ELISA and Western blot testing. Particle Agglutination Western Blot • Most popular confirmatory test. – Utilizes a lysate prepared from HIV virus. – The lysate is electrophoresed to separate out the HIV proteins (antigens). – The paper is cut into strips and reacted with test sera. – After incubation and washing anti-antibody tagged with radioisotope or enzyme is added. – Specific bands form where antibody has reacted with different antigens. – Most critical reagent of test is purest quality HIV antigen. – The following antigens must be present: p17, p24, p31, gp41, p51, p55, p66, gp120 and gp160. Western Blot • Antibodies to p24 and p55 appear earliest but decrease or become undetectable. • Antibodies to gp31, gp41, gp 120, and gp160 appear later but are present throughout all stages of the disease. Western Blot • Interpretation of results. – No bands, negative. – In order to be interpreted as positive a minimum of 3 bands directed against the following antigens must be present: p24, p31, gp41 or gp120/160. • CDC criteria require 2 bands of the following: p24, gp41 or gp120/160. gp160 gp120 p68 p55 p53 gp41-45 Spectrum of anti-HIV testing p40 p34 p24 p18 p12 early DNA PCR RNA PCR p24 Ag 3rd gen ELISA 1st gen ELISA Detuned ELISA 1wk 2wk recent / established advanced 3wk 2mo 6mo 1yr 2yr 3yr +8yr Western Blot • • • • Expensive – $ 80 - 100 technically more difficult visual interpretation lack standardisation – - performance – - interpretation – - indeterminate reactions – resolution of ?? • ‘Gold Standard’ for confirmation Western Blot • Indeterminate results are those samples that produce bands but not enough to be positive, may be due to the following: – – – – – – – prior blood transfusions, even with non-HIV-1 infected blood prior or current infection with syphilis prior or current infection with malaria autoimmune diseases (e.g., diabetes, Grave’s disease, etc) infection with other human retroviruses second or subsequent pregnancies in women. run an alternate HIV confirmatory assay. • Quality control of Western Blot is critical and requires testing with strongly positive, weakly positive and negative controls. Indirect immunofluorescence • Can be used to detect both virus and antibody to it. • Antibody detected by testing patient serum against antigen applied to a slide, incubated, washed and a fluorescent antibody added. • Virus is detected by fixing patient cells to slide, incubating with antibody. Detection of p24 HIV antigen • The p24-antigen screening assay is an EIA performed on serum or plasma. • P24 antigen only present for short time, disappears when antibody to p24 appears. • Anti-HIV-1 bound to membrane, incubated with patient serum, second anti-HIV-1 antibody attached to enzyme label is added (sandwich technique), color change occurs. • Optical density measured, standard curve prepared to quantitate results. Detection of p24 HIV antigen • Positive confirmed by neutralizing reaction, preincubate patient sample with anti- HIV, retest, if p24 present immune complexes form preventing binding to HIV antibody on membrane when added. • Test not recommended for routine screening as appearance and rate of rise are unpredictable. • Sensitivity lower than ELISA. Detection of p24 HIV antigen • Most useful for the following: – early infection suspected in seronegative patient – newborns – CSF – monitoring disease progress Polymerase Chain Reaction (PCR) • Looks for HIV DNA in the WBCs of a person. • PCR amplifies tiny quantities of the HIV DNA present, each cycle of PCR results in doubling of the DNA sequences present. • The DNA is detected by using radioactive or biotinylated probes. • Once DNA is amplified it is placed on nitrocellulose paper and allowed to react with a radiolabeled probe, a single stranded DNA fragment unique to HIV, which will hybridize with the patient’s HIV DNA if present. • Radioactivity is determined. Virus isolation • Virus isolation can be used to definitively diagnose HIV. • Best sample is peripheral blood, but can use CSF, saliva, cervical secretions, semen, tears or material from organ biopsy. • Cell growth in culture is stimulated, amplifies number of cells releasing virus. • Cultures incubated one month, infection confirmed by detecting reverse transcriptase or p24 antigen in supernatant. Viral Load Tests • Viral load or viral burden is the quantity of HIV-RNA that is in the blood. • RNA is the genetic material of HIV that contains information to make more virus. Viral Load Tests • Viral load tests measure the amount of HIV-RNA in one milliliter of blood. • Take 2 measurements 2-3 weeks apart to determine baseline. • Repeat every 3-6 months in conjunction with CD4 counts to monitor viral load ant T-cell count. • Repeat 4-6 weeks after starting or changing antiretroviral therapy to determine effect on viral load. Testing of Neonates • Difficult due to presence of maternal IgG antibodies. • Use tests to detect IgM or IgA antibodies, IgM lacks sensitivity, IgA more promising. • Measurement of p24 antigen. • PCR testing may be helpful but still not detecting antigen soon enough: 38 days to 6 months to be positive. References • http://www.cat.cc.md.us/courses/bio141/lecguide/unit2/viruses/hivlc.html#translat • http://pathmicro.med.sc.edu/lecture/HIV3.htm • http://www.avert.org/hivstages.htm • http://www.aidsinfo.nih.gov/guidelines/ • http://www.hopkins-aids.edu/publications/pocketguide/pocketgd0105.pdf • http://www.modares.ac.ir/sci/saman_h/Pages/applications.htm • http://hivinsite.ucsf.edu/InSite?page=kb-02&doc=kb-02-02-02-02 • http://www.hivandhepatitis.com/recent/test/realtime/061604_f.html