* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Elec Structure of Atom
Double-slit experiment wikipedia , lookup
Planck's law wikipedia , lookup
Atomic orbital wikipedia , lookup
Particle in a box wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Tight binding wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Electron configuration wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Electron scattering wikipedia , lookup
Hydrogen atom wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Matter wave wikipedia , lookup
Wave–particle duality wikipedia , lookup
Atomic theory wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
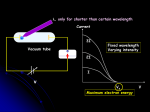
Electronic Structure of Atoms: Chapter 6, Sections 1-4 6.1 The Wave Nature of Light Electronic structure – describes energies and arrangement of electrons around . Electromagnetic radiation – radiant energy – carries energy through space – it moves through a vacuum at the speed of light (3 x 108 m/s). It has electric and magnetic components that are in a wave-like structure. 6.1 The Wave Nature of Light Waves are described in terms of: Wavelength λ: distance between peaks Frequency ν: periods per unit time v λ=c ; c = speed of light 6.2 Quantized Energy and Photons The minimum amount of radiant energy that an object can gain or lose is related to the frequency of the radiation: E=hv. Planck’s constant; h=6.63 x 10-34 J-s. Energy is quantized, meaning it can only have certain allowed values. Einstein proposed that that light behaves as if it consisted of quantized energy packets called photons. Each photon carries energy, E=hv. 6.3 Bohr’s Model of the Hydrogen Atom Spectrum: produced by dispersion of radiation into its component wavelengths. Continuous spectrum: contains all wavelengths. Line spectrum: contains only certain wavelengths. En represents the energy of the hydrogen atom. (n) is the principal quantum number and the energy of the atom increases as n increases. 6.3 Bohr’s Model of the Hydrogen Atom Use the following equation to relate the frequency of absorbed or emitted light and the principal quantum numbers of the two states. ∆E Rh 1 - 1 v = h = h n 2i n 2f Rh is known as Rhydberg’s constant 2.18 x 10-18 J h is Planck’s constant 6.63 x 10 -34 Js Wavelength to frequency _c_ λ= v Be careful that you convert from nm or Angstroms to meters so that you units match. 6.3 Bohr’s Model of the Hydrogen Atom The lowest energy level is achieved in the ground state where n=1. Other n values correspond to excited states. Light is emitted when the electron drops from a high energy state to a low energy state; light can be absorbed to excite the electron from a low energy state to a high energy state. The frequency of light emitted or absorbed must be such that hv=the difference in energy between two allowed states of the atom. 6.4 The Wave Behavior of Matter Radiation appears to have either a wavelike or a particle-like (photon) character Louis De Broglie (1892-1987) suggested that the electron in its movement about the nucleus has associated with it a particular wavelength De Broglie proposed that wavelength of an electron depends on its mass and velocity λ= h/mv; wavelength = Planck’s constant/ momentum (mass x velocity) De Broglie used the term matter waves to describe the wave characteristics of material particles 6.4 The Wave Behavior of Matter German physicist Werner Heisenburg concluded that the dual nature of matter places a fundamental limitation on how precisely we can know both the location and the momentum of any object Heisenburg’s principle is called the uncertainty principle which states that it is inherently impossible for us to know simultaneously both the exact momentum of an electron and its exact location in space Thanks to Alex Kawa, Kate Harkness, Will Lambert, Adam Robinson, Tori Waldron, Ankush Khullar