* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Introduction to Instrumental Analysis and Evaluation of Data
Survey
Document related concepts
Transcript
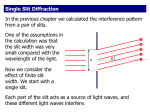
Introduction to optical spectroscopy Chemistry 243 Fundamentals of electromagnetic radiation c c EE hh h Planck's constant 6.626 10 =frequency in Hz (s-1) c speed of light 3.00 108 m 34 J s s =wavelength 1 cm 1 wavenumbers E Electromagnetic spectrum High Energy Low Energy http://www.yorku.ca/eye/spectrum.gif Terminology Spectroscopy is the study of the interaction of light and matter Spectrometry is the establishment of the pattern of interaction (as a function of energy) of light with particular forms of matter NMR or X-Ray spectroscopy; spectroscopist Mass spectrometry (MS); spectrometrist Spectrophotometry is the quantitative study of the interaction of light with matter UV-Visible spectrophotometry (I’ve never heard anyone called a spectrophotometrist) What chemical and/or material properties can we measure using spectral methods? Broad and powerful applications Elemental composition (often metals; CHNO) Identity of a pure substance (what is it?) Components of a mixture (purity?) Amount of a substance in a mixture (how much?) Bulk/major component, minor component, trace component, ultra-trace component Surface composition Material property (stress/strain, polymer crosslinking, change of state, temperature) Reaction rate, mechanism, products What properties of incident or generated light can we measure? Absorption Fluorescence (fast) & Phosphorescence (slow) Thermal Emission Chemiluminescence Scattering Refraction or Refractive Index Polarization, Phase Interference/Diffraction Coherence Chemistry consequent to the above What atomic/molecular properties affect or are affected by light? Rotation (typically refers to a molecule) Vibration (typically refers to a molecule) Electronic Excitation (atomic or molecular) Ionization (loss of electron to yield a cation) Combinations of the above: Rotation-vibration (infrared/Raman) Rotational, vibrational, electron excitation (UV-Vis) Ionization with UV absorbance (strong excitation) The properties you want to study help to select a suitable wavelength High Energy Low Energy Why wavenumber? unit cm 1 c 1 E h hc hc E hc ( ) The energy difference between two wavenumbers is the same regardless of spectral region or λ Wavelength is not proportional to energy; it has a reciprocal relation to energy, so: 1 E The energy difference between two wavelengths (in nm or angstroms) varies as a function of spectral region. Selecting the right optical method Emission Excitation Source Plasma, flame, or chemical Focus Sorting of Energy, Space, and Time Detection Chemiluminescence is emission caused by a chemical reaction. Fluorescence is emission caused by excitation Computer control enhances and optimizes the info extracted from each instrument component. Absorption Transmission and/or Reflection can also occur Focus Specimen Energy, Space, and Time Sorting Focus Detection Nearly linear light path geometry for multi-wavelength, simultaneous light detection Absorbance Light Source Wavelength (λ) Relaxation is non-radiative; sample warms up a bit via vibration and rotation Fluorescence (fast) & Phosphorescence (slow) Light Source Focus Specimen (Laser) May include energy sorting Energy, Space, and Time Sorting Detection Emission Power Focus Typical geometry 90°, but angle variable Radiative Raman Scattering Light Source Focus Specimen Laser Focus Typical geometry 90°, but angle variable Energy, Space, and Time Sorting Detection Same geometrical layout as fluorescence and phosphorescence, … But what happens is not the same as absorption or emission Raman Scattering Elastic scattering: Eex = Eout Inelastic scattering: Ein < Eout and Ein > Eout Eexcitation virtual state virtual state -E Eex +E Different classes of optical spectroscopy Emission Absorbance (UV/Vis or IR) Lamps, LEDs Flame, plasma, chemistry Raman scattering Fluorescence/ Phosphoresence Lamps, LEDs, lasers lasers Classes of light sources Light sources: Common examples Blackbody radiation Light emitting diode (LEDs) Arc lamp/hollow cathode lamp Lasers Solid-state Gas/excimer Dye laser Thermal excitation Combinations (laser to vaporize sample leading to thermal emission) Continuum spectra and blackbody radiation A solid is heated to incandescence It emits thermal blackbody radiation in a continuum of wavelengths High E = Low λ = High T blackbody peak b T Wien’s Law b is Wein’s displacement constant 2.898 106 K nm peak T 2.898 106 K nm roomtemp 9.82 m 295 K 2.898 106 K nm human 9.35 m 310 K Skoog, Fig. 6-22 Continuum spectra and blackbody radiation T ≈ 1200° C T ≈ 1473 K http://en.wikipedia.org/wiki/Image:Blackbody-lg.png http://en.wikipedia.org/wiki/Black_body Continuum sources Common sources Deuterium lamp (common Ultraviolet source) Ar, Xe, or Hg lamps (UV-vis) Not always continuous; spectral structure possible http://www1.union.edu/newmanj/lasers/Light%20Production/LampSpectra.gif http://creativelightingllc.info/450px-Deuterium_lamp_1.png Light emitting diodes (LEDs) First practical visible region LED invented by Nick Holonyak in 1962 (GE; UIUC since 1963) “Father of the light-emitting-diode” An LED is a semiconductor which emits electroluminescence http://en.wikipedia.org/wiki/Nick_Holonyak http://upload.wikimedia.org/wikipedia/commons/7/7c/PnJunction-LED-E.PNG http://www.pti-nj.com/images/TimeMasterLED/LED-spectra_remade.gif Light emitting diodes (LEDs) Cheap, low energy, long-lasting, small, fast Commonly used in display screens, stoplights, circuit boards as state indicators Lots of colors Infrared LEDs used in remote controls http://en.wikipedia.org/wiki/File:Verschiedene_LEDs.jpg Line (emission) sources Continuous wave Pulsed Hollow cathode discharge lamp Microwave discharge Flames and argon plasmas Pulsed hollow cathode Spark discharge All these are non-laser A line source is a light source that emits at a narrow wavelength called an emission “line” Lasers Light Amplification by Stimulated Emission of Radiation • Intense light source • Narrow bandwidth (small range λ < 0.01 nm) • Coherent light (in phase) Lasers Light Amplification by Stimulated Emission of Radiation • • • • Pumping Spontaneous Emission Stimulated Emission Population Inversion Laser design A photon cascade! Skoog, Fig. 7-4 Lasing medium is often: • a crystal, like ruby • a dye solution • a gas or plasma Pumping Generation of excited electronic states by thermal, optical, or chemical means. Skoog, Fig. 7-5 Spontaneous emission or relaxation Random in time No directionality Monochromatic (same λ), but incoherent (not in phase) Solid vs. dashed line – 2 different photons Skoog, Fig. 7-5 Stimulated emission The excited state is struck by photons of precisely the same energy causing immediate relaxation Emission is COHERENT Emitted photons travel in same direction Emitted photons are precisely in phase Skoog, Fig. 7-5 Population inversion When the population of excited state species is greater than ground state, an incoming photon will lead to more stimulated emission instead of absorption. Normal population distribution Pexcited < Pground Inverted population Pexcited > Pground Skoog, Fig. 7-6 3- and 4-state lasers Population inversion easier in 4-state system Things stack up here. Population inversion easily achieved. Population relatively low down here Skoog, Fig. 7-7 Laser design A photon cascade! Skoog, Fig. 7-4 Lasing medium is often: • a crystal, like ruby • a dye solution • a gas or plasma Continuous wave laser sources Nd3+:Yttrium aluminum garnet (YAG: Y3Al5O12) Solid state 1064 nm, 532 nm, 355 nm, 266 nm The GTE Sylvania Model 605, uses a Nd-YAG laser rod set in a "double elliptical“ reflector, is pumped by two 500-W incandescent lamps, and is limited to a low order mode by an aperture in the laser cavity. Continuous wave laser sources Helium-Neon (HeNe) Gas, but emission comes from generated plasma (very excited state atoms) 632.8 nm, 612 nm, 603 nm, and 543.5 nm; 1.15 & 3.39 μm Emission lines all the way out to 100 μm 99% reflective 99.9% reflective Continuous wave laser sources Ar+ Gas laser, but emission comes from ions Uses lots of electrical power to generate ions 351.1 nm, 363.8 nm, 454.6 nm, 457.9 nm, 465.8 nm, 476.5 nm, 488.0 nm, 496.5 nm, 501.7 nm, 514.5 nm, 528.7 nm, 1092.3 n Coherent Innova 90 Up to 5 W of output! ~100x my laser pointer Other continuous wave laser sources Cu vapor 520 nm HeCd 440 nm, 325 nm Dye lasers Pulsed lasers sources Nd:YAG Ti:sapphire Solid state—often pumped by Nd:YAG Tunable output aroudn 800-1200 nm Produces femtosecond pulses Nitrogen Solid state Often nanosecond pulses 1064 nm, 532 nm, 355 nm Gas 337 nm Excimer lasers (gas mixtures; excited state is stable) Tunable dye lasers (λ is selective within limits) Laser diodes Band gap energy, Eg Resonant Cavity emits At 975 nm Used in CD and DVD players (not very strong) Wavelengths now available from IR to near UV regions Skoog, Figs. 7-8 & 7-9. Tip going forward Keep your variables straight v for velocity or for frequency Microsoft equation editor gives: nu (1/s) v vee (m/s) I will use m for integer, textbook uses n Easy to get mixed up with refractive index, n Properties of electromagnetic radiation Transmission Refraction Reflection Scattering Optical Components Interference Diffraction Properties of electromagnetic radiation y A sin t 2 y A sin 2t y A ν φ ω = = = = = magnitude of the electric field at time t ymax – also called the amplitude of y frequency in s -1 (cycles per second) phase angle (an offset relative to a reference sine wave) angular velocity in radians/sec (a handy definition) Recall: π radians = 180 degrees Interference – magnitudes add or subtract Constructive Interference - In Phase 2 1 0 A Amplitude -1 -2 B -3 -4 -5 A+B B is in phase with A -6 -7 0 2 4 6 Time 8 10 12 Interference – magnitudes add or subtract Destructive Interference - Half Wave Shift 2 1 0 A Amplitude -1 B -2 -3 -4 A+B -5 B is 180 degrees (π radians) shifted from A -6 -7 0 2 4 6 Time 8 10 12 Interference – magnitudes add or subtract Interference -- Quarter Wave Shift 2 1 0 A B Amplitude -1 -2 -3 A+B -4 -5 -6 -7 0 2 4 6 8 Time B is 90 degrees (π / 2 radians) shifted from A 10 12 Interference between waves of different frequency Wave 1 + 2 1 2 Skoog, Fig. 6-5 Transmission through materials Compared to vacuum, the velocity of light is reduced when propagating through materials that have polarizable electrons. Wavelength also decreases All electrons are polarizable to some extent c ni vi E h constant c medium Skoog, Fig 6-2. cvacuum = 2.99792 x 108 m ● s-1 Index of Refraction Refractive index is measure of how much light is slowed: c ni vacuum refractive index at a given wavelength ( 1) vi vi velocity at a given wavelength Refractive index is wavelength- and temperaturedependent for many materials: Material n @ 589.3 nm Vacuum (air) 1.00 Water 1.33 nwater vs Temperature Wavelength-dependence of nSiO2 1.336 1.334 Hexadecane 1.43 Quartz 1.46 Toluene 1.49 Glass 1.58 refractive index 1.332 1.33 1.328 1.326 1.324 1.322 1.32 1.318 1.316 0 (light flint) 20 40 60 o temperature ( C) http://www.rp-photonics.com/refractive_index.html 80 100 Refraction Snell’s law: sin 1 n2 v1 sin 2 n1 v2 Oil immersion lenses for high magnification microscopy Medium 1 Medium 2 Here, n2 > n1 Velocities, not frequencies Skoog, Fig. 6-10 For your information … Book Error on page 141, equation 6-12: sin 1 n2 v1 velocity in medium 1 sin 2 n1 v2 velocity in medium 2 This is correct: Snell’s Law of Refraction Reflection Amount of loss at a reflection increases with refractive index mismatch. For right angle light entrance into a medium: 2 n2 n1 Ir reflected intensity 2 incident intensity I0 n2 n1 Reflective loss is angle-dependent Fresnel equations (which we will skip) Most important case is: total internal reflection 1 Total internal reflection Light incident upon a material of lesser refractive index is bent away from the normal so that the exit angle is greater than then incident angle. 2 nentry > nexit θ2 > θ1 At the critical incident angle, the exit angle is 90° - beam does not exit Angles larger than the critical incident angle lead to total internal refection (TIR) Medium2 Medium1 nexit nentry n1 sin 1 n2 sin 2 nentry sin 1 nexit sin 2 nentry sin critical nexit sin( 90) nentry sin critical nexit Modified from Skoog 1 When this is true, θ1 = critical entry angle for TIR Total internal reflection nentry sin critical nexit When this is true, θ1 = critical entry angle for TIR. If θ1 > θcritical result is TIR. TIR fluorescence microscopy: Evanescent wave samples a very narrow slice of the sample very near to the dielectric interface Typically ~200 nm http://hyperphysics.phy-astr.gsu.edu/Hbase/phyopt/totint.html http://www.olympusmicro.com/primer/techniques/fluorescence/tirf/tirfintro.html Total internal reflection nentry sin critical nexit When this is true, θ1 = critical entry angle for TIR. If θ1 > θcritical result is TIR. TIR fluorescence microscopy: Good for studying adhered cells; low background http://hyperphysics.phy-astr.gsu.edu/Hbase/phyopt/totint.html http://www.olympusmicro.com/primer/techniques/fluorescence/tirf/tirfintro.html Fiber optics Extruded strands of glass or plastic that guide light via total internal reflection. Core has higher refractive index than cladding. Flexible Material choice allows transmission in UV, visible, or IR Skoog, Fig 7-39. Follows all the rules of Snell’s Law Scattering Raman scattering Inelastic scattering Rayleigh scattering offset from by frequency of molecular vibrations Molecules or aggregates smaller than Intensity ~ 1/4 Mie scattering Particles large (or comparable) to Used for particle sizing Essential optical elements Lenses Mirrors Prisms Filters Gratings Basic optical components Mirrors Prisms Reflection Refraction Concave mirror is converging Snell’s Law Convex mirror is diverging n1 sin 1 n2 sin 2 Filters Absorption filters Cheap, visible region; colored glass Cutoff filters – long-pass short-pass Interference filters 2d cos n d = thickness of dielectric layer n = refractive index of dielectric medium m air 2dn m cos mm = integer interger air θ is usually zero so, cos θ = 1. Also, m is usually 1 ʹ = wavelength in the dielectric material Skoog, Fig. 7-12 Interference filters Almost monochromatic Bandwidth of a filter is width at half-height (aka full-width @ half-max) Skoog, Fig. 7-13 Diffraction of coherent radiation: Interference at work Consequence of interference d = distance from slit B to C m d sin m integer constructive destructive constructive destructive (m is the order of interference) constructive Distance x to y is one λ m is: • 0 for E • 1 for D Skoog, Figs. 6-7, 6-8 m used here, text uses n Diffraction of coherent radiation: Interference at work Consequence of interference m d sin m integer (m is the order of interference) m d sin Skoog, Fig. 6-8 BC DE OD m used here, text uses n You can now determine the wavelength of light based on things that are easy to measure! Monochromators Used to spatially separate different wavelengths of light: prisms, gratings Bunsen prism monochromator Czerny-Turner grating monochromator Skoog, Fig. 7-18 Gratings and monochromators Reflection + diffraction: echellette-type grating m CB BD CB d sin i BD d sin r m d sin i sin r The condition for constructive interference. The m = 1 line is most intense. Skoog, Fig. 7-21 The surface contains “grooves” or “blazes”. Take a look at Example 7-1, Page 184. Monochromators Used to spatially separate different wavelengths of light: prisms, gratings Czerny-Turner grating monochromator Skoog, Fig. 7-18 Useful metrics for monochromators Dispersion (page 185); high dispersion is good Integration of m d sin i sin r at constant i gives the angular dispersion: dr m d d cos r r = angle of reflection d = distance between blazes Linear dispersion, D, is the variation of λ along the focal plane position, y: dy f dr D d d f focal length A measure of the ability to separate wavelengths Reciprocal linear dispersion, D-1: D 1 d d cos r d for small r dy mf mf More useful, results in D-1 in nm per mm or similar Useful metrics for monochromators—continued Resolving power (R; unitless) Limit of monochromator’s ability to distinguish between adjacent wavelengths. R mN (unitless) N Number of grating blazes illuminate d Light gathering power (f-number, F; unitless) Collection efficiency—improve for maximizing S/N Efficiency scales as the inverse square of F F f d f focal length of collimating mirror or lens d diameter of collimating optic d E f 2 Complications with monochromators Overlap of orders m = 1, = 600 nm and m = 2, = 300 nm spatially overlap You can get ’s mixed up if light source contains many wavelengths Additional wavelength selection often needed Filter, prism, detector λ selection device, digital analysis after data collection, background subtraction Might need to use a different light source if your wavelength of interest is not “clean” Slit width and spectral resolution of a spectrometer Tradeoff exists between sensitivity and resolution High intensity = high sensitivity (low noise) Two basic concepts: If you make the entrance slit width too big, you let in a lot of light (that’s good – high intensity), but it can be multiwavelength; a large section of light dispersed in is let in If you make the entrance slit width too small, you let in less light (less intensity), but its range is smaller Poor light intensity, good spectral resolution Entrance slit (creates image) and exit slit (output filter) Good light intensity, poor spectral resolution Usually the same width Optimal slit width based upon grating dispersion Slit width For just passing 2 Skoog, Fig. 7-24 Slit width Both entrance and exit slits narrowed from top to bottom Ptotal ∆𝑒𝑓𝑓 = 𝑤𝐷 −1 w is slit width If spectral bandwidth is /2, good spectral resolution Skoog, Fig. 7-25 Slit width Watch the effect of adjusting the slit width and the resultant spectral bandwidth on the following data sets of benzene vapor. −1 ∆𝑒𝑓𝑓 = 𝑤𝐷 w is slit width Skoog, Fig. 7-26 Optical Photodetectors More sensitive Less sensitive A. B. C. D. E. F. G. H. I. Photomultiplier tube (PMT) CdS photoconductivity GaAs photovoltaic cell CdSe photoconductivity cell Se/SeO photovoltaic cell Si photodiode PbS photoconducitivity Thermocouple Golay cell These generally make current or voltage when light hits them. Ideal photodetector (photon transducer) High sensitivity High S/N Fast response time Signal directly proportional to # of photons detected Zero dark current The blank is zero S Photons counted N Or, equivalently, S kP Ideal photodetector (photon transducer) High sensitivity High S/N Fast response time Signal directly proportional to # of photons detected Zero dark current The blank is zero S kP Here’s what really happens: S kP kdark Signal is Function of λ Constant dark current term (non-zero) Three main photodetector types Photon transducers (directly “count” photons) Charge transfer devices Photomultiplier tubes (PMTs) Charge injection devices (CID) Charge coupled devices (CCD) Thermal transducers Photons strike the transducer Temp increases Temp increase increases conductivity Current or voltage are measured Vacuum phototube Cathode is coated with photo-emissive material Emitted electrons are collected anode. # of electrons is directly proportional to # of photons. Current is easy to amplify. Usually have small dark current. Operate at ~ 90V bias Not so portable Skoog, Fig. 7-29. Photomultiplier tube (PMT) # of electrons is amplified by photoelectric effect upon acceleration towards dynodes Each dynode biased ~ 90V more positive than previous dynode (or cathode) Voltage drop accelerates electrons to dynode cascade Amplification: 106-107 electrons per incident photon; electron cascade http://www.nt.ntnu.no/users/floban/KJ%20%203055/PMT.jpg Photomultiplier tube (PMT) Advantages: Very sensitive in UV-Vis region, single photon sensitivity Cooled PMT has very low background (kdark approaches zero) Linear response Fast response Disadvantages Easily damaged by intense (ambient) light Noise is power dependent Single channel: can’t use for imaging Photovoltaic cell Light strikes a semiconductor (Se) and generates electrons and holes Magnitude of current is proportional to # of photons Requires no external power supply! Disadvantages: hard to amplify signal and fatigue (wears out) Useful for portable analyses, field work, outdoor setting Skoog, Fig. 7-28 Photodiodes (Silicon 190-1100 nm, InGaAs 900 – 1600 nm) Reverse-biased p-n junction Conductance goes to near zero Photons create electron hole pairs that migrate to opposite contacts and generate current Battery powered Portable applications Are not as prone to some electronic noise sources 60 Hz line noise Skoog, Fig. 7-32 Multichannel transducers Allow simultaneous interrogation of multiple wavelengths Imaging Photodiode arrays (1-D array) Charge-transfer devices (2-D array) Charge-injection devices Charge-coupled device (CCD) CMOS Photodiode arrays Each diode has defined spatial address Advantages Disadvantages Multichannel (used for imaging) More robust than PMT Not as sensitive as PMT Slower response time Common in cheaper UV-Vis instruments Often perfectly adequate Skoog, Fig. 7-33 Charge transfer devices Converts light into charge Negative-biasing leads to increased capture of holes under pixel electrodes Photon ejects electron and the device collects and stores charges Potential well 105-106 charges per pixel Configured as CID or CCD Schematic is for CID Skoog, Fig. 7-35 Charge transfer devices (continued) Charge-injection device (CID)-measures accumulated voltage change (nondestructive read; persistent after read) Charge-coupled device (CCD)-moves accumulated charges to amplifier and readout (destructive read; gone after read) Measurements can be made while integrating Very high sensitivity; 104-105 pixels High resolution spectral imaging Complementary metal oxide semiconductor (CMOS) Webcam technology: CHEAP! OK sensitivity, large pixel density CCD (charge coupled device) Pixels read one at a time by sequential transfer of accumulated charge From: “CCD vs. CMOS: Facts and Fiction” by Dave Litwiller, in Photonics Spectra, January 2001 CMOS detectors Digital camera and webcam technology Each pixel can be read individually Image from Wikipedia From: “CCD vs. CMOS: Facts and Fiction” by Dave Litwiller, in Photonics Spectra, January 2001 CCD Essentially serial Each pixel read one at a time by common external circuitry CMOS Voltage conversion and buffering Outputs an analog signal Historically gave higherresolution images Relatively expensive Essentially parallel Each pixel has its own red out circuitry “on-chip” Outputs a digital signal Reduced area for light absorption Relatively inexpensive High power consumption Up to 100x more than CMOS Allows amplification and noise correction More susceptible to noise Highly commercialized fab Runs on less power Requires less “off-chip” circuitry Both approaches exist today Photoconductivity transducers Semiconductors whose resistance decreases when they absorb light Absorption promotes electron to conduction band. Useful in near IR( = 0.75 to 3 m) Cooling allows extension to longer wavelengths by reducing thermal noise Thermal transducers Solution for IR region (low energy photons) Thermocouples Bolometer (thermistor) Light absorbed heats the junction (two pieces of dissimilar metal) which leads to a change in voltage relative to a reference thermocouple. Material changes resistance as a function of temp Pyroelectric devices Temperature-dependent capacitor Change in temperature leads to change in circuit current