* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Graphite
Survey
Document related concepts
Transcript
ME551/GEO551 Geology of
Industrial Minerals
Spring 2007
Commodities, Part 2
Clays, Diamonds, Diatomite,
Fluorite, Garnet, Graphite
Reminders
• What is trap rock?
• Field trip to potash mines next week
–
–
–
–
Monday leave at 9 AM
Tues tour and return to Socorro
Hotel
Buckets for collecting
• Term Projects?
• March 20 I will not be teaching—Jim Barker
• Any questions on the midterm?
– Due March 9
Clays
• Bentonite—Jeremy
Clays—Introduction
• Stone age
• Types
– ball clay (primarily of kaolinite with illite,
chlorite, smectite minerals, quartz)
– bentonite (smectite with feldspars, biotite,
quartz)
– common clay (illite and chlorite, others)
– fire clay (kaolinite, halloysite, diaspore)
– fuller’s earth (attapulgite, montmorillonite)
– kaolin
Types
• layer silicates
– layers of tetrahedral and octahedral sheets
– Kaolinite, smectite, illite, chlorite, vermiculite
• the metal oxides and hydroxides and oxy-oxides
– gibsite
• amorphous and allophanes
– structurally disordered aluminosilicates
– Allophane, Imogolite
Clays—definition
• particle size of less than 2 micrometers
• family of minerals
• rock term
Clays—properties
• chemical composition
• layered structure
• size
• great affinity for water (double in thickness
when wet)
• soak up ions, release the ions later when
conditions change
Clays—properties
•
•
•
•
•
•
Color
plasticity
mineral composition
absorption qualities
firing characteristics
clarification properties
d
e
p
e
n
d
e
n
t
.
Properties—charge sources
I
• Two main sources of charge
in clay
n
minerals are isomorphous
a substitution and
pH-dependent charges. l
l
o
p
h
a
n
e
s
,
http://jan.ucc.nau.edu/doetqp/courses/env320/lec12/Lec12.html
Charge properties
• Charge development of on silicate clays is mainly due to
isomorphous substitution.
• This is the substitution of one element for another in ionic
crystals with out change of the structure.
• It takes place during crystallization and is not subject to
change afterwards.
• It takes places only between ions differing by less than
about 10% to 15% in crystal radii.
• In tetrahedral coordination, Al3+ for Si4+ and in
octahedral coordination Mg2+, Fe2+, Fe3+ for Al3+.
• Charges developed as a result of isomorphous substitution
are permanent and not pH-dependent.
Charge properties
• In allophanes, some silicate clays e.g. kaolinite,
and the metal oxides the main source of charge are
termed pH -dependent charges because these
charges depend on the pH of the soil.
• pH depend charges are variable and may either be
positive or negative depending on the pH of the
soil.
• In the metal oxides acid soils tend to develop
positive charges because of the protonation of the
oh ggoud on the oxide surfaces.
Clay—uses
• Ceramics
• fillers and extenders
• construction (hydraulic cement, structural
clay products, aggregates)
• drilling mud
• fiberglass
• Iron Ore Pelletizing
• paper
• carrier to mix paint and color pigment
Ball clay—uses
• Burn to a light color and accepts glaze,
plastic
• 35% floor and wall tile
• 22% sanitaryware
• 43% other uses
Bentonite—uses
• Clay consisting of smectites
Common clay—uses
•
•
•
•
56% brick
20% cement
16% lightweight aggregate
8% other uses (fillers and extenders)
Fire clay—uses
• 73% refractories
• 27% other uses
Fuller’s earth—uses
• mineral substance characterized by the
property of absorbing basic colors and
removing them from oils
• fulling of wool to remove oil and grease
• 75% absorbent uses
• 25% other uses
Kaolin—uses
• Near white containing kaolinite
• 55% paper
• 7% refractories
• 38% other uses
Kaolin—uses
•
•
•
•
•
•
•
•
•
•
•
mildew-resistant latex paints
vinyl wire insulation
printing inks
Cosmetics
rubber tires
fiberglass and nylon
auto and truck body components
production of medicines
ceramics
catalysts for petroleum refining
extenders for fertilizers, pesticides, and herbicides
Kaolin
Clays—substitutions
• Limited substitutions possible
• calcium carbonate
• talc
Clays—production
– ball clay
– common clay: various
– fire clay
– fuller’s earth: U.S., Germany
– kaolin: U.S., Uzbekistan, Czech Republic,
United Kingdom, Brazil
Clays—geology
• soil horizons
• continental and marine sediments
• geothermal fields
• volcanic deposits
• weathering rock formations
• coal beds
Bricks—processing
• Common clay used to make bricks
• formed or shaped either by extrusion
– involves forming a column of clay by
pushing the material through a die at high
pressure.
– then cut into bricks (known as 'wirecut')
– drainage pipes and clay roof tiles made
similar process
Bricks—processing
• or the 'soft-mud' process
– individual bricks are formed in a sand-lined
mould from a clay with a relatively high
moisture content (known as 'stock' bricks)
– dried prior to firing
– fired using natural gas in a linear kiln
known as a 'tunnel kiln’
– 1050–1100°C
Environmental considerations—clay
• Open pits
• organic emissions (EPA developing
standards, MACT)
• impoundment of slimes
• dust control
Colin C. Harvey, 1999
Diamonds
Diamonds
• Greek
adamas
meaning
invincible
• Used in
India 2,500
yrs ago
Diamonds—introduction
• clarity, color, shape, size is used as
industrial-grade diamond (nongem)
http://www.brysonburke.com/diamonds_find_the_source.html
Diamonds—properties
• Hardest substance known
• highest thermal conductivity
• chemical stability
• optical properties
• refract light
atomic connectivity of the carbon atoms gives the
gem its hardness
Diamonds—production
Diamonds—uses
•
•
•
•
•
•
•
•
•
•
Middle Ages--healing powers
Grinding
drilling
cutting
polishing
abrasive
wear- and corrosion-resistant coatings,
special lenses
heat sinks in electrical circuits
wire drawing
Diamonds—substitutions
•
•
•
•
cubic boron nitride
silicon nitride
but diamond is more than twice as hard
synthetic diamonds (US)
Diamonds—geology
•
•
•
•
Kimberlites
lamprorites
alluvial (placer) deposits for these rocks
molten rock from 75 to 120 miles below the
earth's surface 40 kbar and 900° C
The slightly misshapen octahedral shape of this rough diamond
crystal in matrix is typical of the mineral. Its lustrous faces also
indicate that this crystal is from a primary deposit
http://en.wikipedia.org/wiki/Diamond
Schematic diagram of a volcanic pipe
http://en.wikipedia.org/wiki/Diamond
Indicator minerals
•
•
•
•
•
•
ilmenite
titanium and magnesium rich chromite
chrome diopside
magnesium rich olivine
pyrope garnets
eclogitic garnets
Carat
• carat weight measures the mass of a
diamond
• One carat is defined as a fifth of a gram
• 200 milligrams
• approximately 0.007 ounce
• point unit—equal to one one-hundredth of a
carat (0.01 carat, or 2 mg)
Price
Mining
Alluvial mining by
traditional
methods
continues,
as seen here in
Sierra Leone.
Mining
Diamonds—processing
• Crush
• scrubbers and degritting and sanding
sections remove fine waste material for
disposal
• Heavy-medium separation or grease belts
• X-ray fluorescence sorters are used to
extract the diamonds
Diatomite
Diatomite—introduction
• made of plant fossils shaped like soda
straws
• silica
• looks like chalk (CaCO3)
• diatomaceous earth
Diatomite
http://www.rockdetective.org/f...
Diatomite
http://www.maidenwell.com/
Chemical composition
•
•
•
•
86% silica
5% sodium
3% magnesium
2% iron
Diatomite—properties
• Light weight (hollow fossil shells)
• does not conduct heat
Diatomite—uses
•
•
•
•
•
•
•
•
•
•
Once used in dynamite
insulate steam pipes
filtration aid (swimming pools)
mild abrasive
mechanical insecticide (physico-sorptive
properties)
absorbent for liquids
Cat litter
activator in blood clotting studies
thermal insulator
plants
Diatomite—substitutions
•
•
•
•
•
•
•
Expanded perlite
silica sand
talc
ground silica sand,
ground mica
clay
exfoliated
vermiculite
•
•
•
•
•
•
•
Perlite
vermiculite
ground limestone
various clays
special brick
mineral wool
expanded perlite
Diatomite—production
Diatomite—geology
• Saltwater
– contains a high crystalline silica content
• Fresh water lake
– dry lakebeds and is characteristically low in
crystalline silica content
Diatomaceous earth
http://www.minerals.epcorp.com..
Dredging is one mining method
http://www.hi.is/HI/Stofn/Myva...
Safety
• drying of the hands, if handled without
gloves
• highly crystalline form of silica, resulting in
sharp edges
• dangerous to breathe and a dust mask is
recommended when working with it
• silicosis
Fluorite
Fluorite
• Latin fluo, meaning flow
Fluorite—introduction
•
•
•
•
•
•
CaF2, Calcium Fluoride
halide
variable color
Luster is vitreous.
transparent to translucent.
Cleavage is perfect in 4 directions forming
octahedrons.
• Hardness is 4
• Fracture is irregular and brittle.
• Specific Gravity is 3.1+ (heavy)
Fluorite—properties
• fluorospar
• ability as a flux
• ore of F
Fluorite—uses
•
•
•
•
•
•
flux in steel and aluminum processing
in the preparation of glasses and enamels
manufacture of hydrofluoric acid
for carved ornamental objects
fluorinated water
gemstone
Fluorite
http://mineral.galleries.com/minerals/halides/fluorite/fluorite.htm
Fluorite—substitutions
• Olivine
• dolomitic limestone
• Byproduct fluorosilicic acid
Fluorite—production
Fluorite—geology
•
•
•
•
•
Rio Grande Rift (RGR) deposits
Mississippi Valley type (MVT) deposits
Sedimentary stratiform deposits
volcanic massive sulfide deposits
gangue in epithermal and mesothermal
veins
Garnet
Garnet
• Latin granatus (“grain")
• possibly a reference to the Punica granatum
("pomegranate"), a plant with red seeds
similar in shape, size, and color to some
garnet crystals
Garnet—introduction
• group of complex silicate minerals with
similar crystalline structures
• A3B2(SiO4)3, where A can be Ca, Mg,
Fe, Mn; B can be Al, Cr, Fe, Ti
Garnet—introduction
• aluminum garnets
– almandine or almandite
– pyrope
– grossularite
– spessartite
• iron garnets
– andradite
• chromium
– uvarovite
Garnet—properties
•
•
•
•
•
Various colors
isometric
specific gravity 3-4
Luster is vitreous
Hardness is 6.5 - 7.5
Almandine
Andradite
http://en.wikipedia.org/wiki/Garnet
Garnet—uses
•
•
•
•
•
waterjet cutting, 35%
abrasive blasting media, 30%
water filtration, 15%
abrasive powders, 10%
other end uses, 10%
Garnet—substitutions
•
•
•
•
natural and manufactured abrasives
Ilmenite
magnetite
plastics
Garnet—production
Garnet—geology
• Gneisses and schists
• contact-metamorphic deposits in
crystalline limestones
• pegmatites
• igneous rocks
• serpentinites
• vein deposits
• alluvial garnet
Graphite
Graphite
• Greek (graphein): to draw/write
• for its use in pencils
Graphite—introduction
• C
• confused with molybdenite, which is denser
and has a silver blue streak
• gray streak
• Luster is metallic to dull
• Cleavage is perfect in one direction
History
• First use of graphite: primitive man to make drawings,
and by Egyptians to decorate pottery.
• Graphite processing: 1400 AD in the Haffnerzell
District of Bavaria.
• Through the Middle Ages graphite was confused with
galena and Molybdenite.
• First names: Plumbago (lead -silver) & black lead
• Discovered: 1565 by Gessner (recognized as a
mineral), but its composition was determined in 1779
by Scheele.
Graphite—properties
• Milled, drilled and turned in a lathe to a desired
shape
• Making Brushes
• conductive
• chemically stable
• high strength
• hardness 1-2
• specific gravity 2.2
• good conductor of electricity
• lubricant
Physical Characteristics
•
•
•
•
•
•
•
•
•
•
•
•
Color is dark gray, black, or black silver.
Luster is metallic to dull.
Transparency crystals are opaque
Crystal System is hexagonal
Hardness is 1 - 2
Specific Gravity 2.2
Cleavage is perfect in one direction.
Fracture is flaky.
Streak is black gray to brownish gray.
Melting Point of 3,500ºC.
Graphite is an excellent conductor of heat and electricity.
Other Characteristics: thin flakes are flexible but inelastic, mineral
can leave black marks on hands and paper.
• Best Field Indicators are softness, luster, density and streak.
Mineralogy
Graphite is a native element composed only of carbon. It has the same composition as
diamond, however it has very different structures.
•Diamond crystallizes in the Isometric system X graphite crystallizes in the hexagonal
system.
Source- http://www.chem.ox.ac.uk/icl/heyes/structure_of_solids/Lecture1/Lec1.html
Graphite
http://www.phy.mtu.edu/faculty/info/jaszczak/borrowdale.html
Graphite—uses
• Refractory applications 45% (brick and
linings)
• brake linings 20%
• lubricants, 5%
• dressings and molds in foundry
operations, 5%
• other uses 25%
END-USES
Main uses are in refractors,
lubricants, brake linings, foundry
moulds, and electrodes. Nontraditional applications include
expanded graphite and graphite
foils (a thin graphite cloth).
Uses of natural graphite in 2004
refractory applications
24%
Graphite Foils
brake linings
46%
foundry operations
13%
8%
9%
lubricants
steelmaking and other uses
(pencils, battery...)
Graphite Packing Expanded Graphite
Graphite—substitutions
•
•
•
•
•
graphite powder
scrap from discarded machined shapes
calcined petroleum coke
Molybdenum disulfide
Finely ground coke with olivine
Graphite—production
Graphite—geology
Types of Natural Graphite :
Disseminated flake
Crystalline vein (lump or high crystalline graphite)
Amorphous
Graphite occurs in many types of igneous, sedimentary & metamorphic rocks.
The more important are those found in metasomatic –hydrothermal
deposits, & in sedimentary rocks that have been subjected to regional or
thermal metamorphism.
Associated Minerals include quartz, calcite, micas, iron meteorites, and
tourmalines.
Geology
Flake graphite:
•
•
•
•
•
is found in metamorphic rocks uniformly distributed through the ore
body or in concentrated lens shaped pockets.
Graphite flake occurs as a scaly or lamella form in certain
metamorphic rocks such as limestone, gneisses and schists.
Carbon concentrations vary between 5% and 40%.
Flake graphite occurs in most parts of the world. Notable deposits are
Canada, Brazil, Madagascar, Australia, USA(Texas-1980, Alabama
&Pennsylvania-1960’s), Germany
Flake: marble, gneiss, and schist (most common rock types)
Source -http://www.alibaba.com/catalog/10876290/Natural_Flake_Graphite.html
Geology
Crystalline vein graphite:
•
is believed to originate from crude oil deposits that through time,
temperature and pressure have converted to graphite.
• Vein graphite is found along the intrusive contacts of pegmatites with
limestone.
• The vein fissures are typically between 1cm and 1 m thick, and are
normally > 90% pure.
• Although this form of graphite is found all over the world, it is only
commercially mined in Sri Lanka.
Source - http://www.asbury.com
Geology
Amorphous graphite:
• Amorphous graphite is found as minute particles in beds of
mesomorphic rocks such as coal, slate or shale deposits.
• The graphite content ranges from 25% to 85% dependent on the
geological conditions.
• Most of the amorphous deposits with economic importance are
formed by metamorphism of coal or carbon rich sediments.
• Notable occurrences are in Mexico, North Korea, South Korea and
Austria.
Source - http://kuroko.mus.akita-u.ac.jp/sampimag/11767e.htm
Artificial Graphite
•
Synthetic graphite can be produced from
coke and pitch.
•
Synthetic Graphite consists mainly of
graphitic carbon that has been obtained by
graphitisation, heat treatment of nongraphitic carbon, or by chemical vapour
deposition from hydrocarbons at
temperatures above 2100K .
•
Synthetic Graphite tends to be of higher
purity though not as crystalline as natural
graphite.
•
On the whole, synthetic graphite tends to be
of a lower density, higher porosity and
higher electrical resistance.
•
Its increased porosity makes it unsuitable
for refractory applications.
Source - http://www.intertrade.com.
Mining Method
Graphite is commonly extracted through open-pit methods. In some
cases, it has been extracted through underground mining (vein deposits
in Sri Lanka).
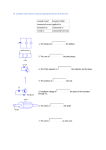
Mining - Graphite ore is extracted with the use of shovels &
bulldozers that load dump trucks with the crude ore.
Primary Crusher
Mill
Flotation Cells
Dryers
Mechanical concentration - The ore is crushed by a primary crusher
and then submitted to a series of roll crushers and classifiers to remove
the oversizes and gangue. Flotation is used for the
mechanical separation of the graphite from impurities present in
the ore. The cycle mill-flotation is repeated until a grade between 87 96% of carbon is reached.
Chemical concentration - Concentration with the use of chemical
agents is used to remove impurities that remain in the graphite after
the mechanical concentration process. Some firms make high purity
graphite (98% - 99%carbon) by leaching concentrate with strong acids
or alkalis.