document
... near old river beds. How do you suppose the diamonds got there? Believe it or not, diamonds have even been found in meteorites from out space! o ...
... near old river beds. How do you suppose the diamonds got there? Believe it or not, diamonds have even been found in meteorites from out space! o ...
Graphite
... • mineral substance characterized by the property of absorbing basic colors and removing them from oils • fulling of wool to remove oil and grease • 75% absorbent uses • 25% other uses ...
... • mineral substance characterized by the property of absorbing basic colors and removing them from oils • fulling of wool to remove oil and grease • 75% absorbent uses • 25% other uses ...
Gemmology Course Outline Lewton-Brain ©86/95/10
... trigonal) are uniaxial and have a single direction (not a line but an entire direction) of single refraction in the doubly refractive (anisotropic or birefringent) crystal. The orthorhombic, monoclinic and triclinic systems are biaxial and have two directions of single refraction in the double refra ...
... trigonal) are uniaxial and have a single direction (not a line but an entire direction) of single refraction in the doubly refractive (anisotropic or birefringent) crystal. The orthorhombic, monoclinic and triclinic systems are biaxial and have two directions of single refraction in the double refra ...
DIAMONDS AND ASSOCIATED HEAVY MINERALS IN
... considered to be preferentially disaggregated on sampling and transport to the surface because of the presence of a carbonate-rich phase that dissociates in response to pressure reduction while temperature remains relatively high (Boyd and Gurney, 1982; Wyllie et al., 1983). The constituent minerals ...
... considered to be preferentially disaggregated on sampling and transport to the surface because of the presence of a carbonate-rich phase that dissociates in response to pressure reduction while temperature remains relatively high (Boyd and Gurney, 1982; Wyllie et al., 1983). The constituent minerals ...
Chapter 7 Theoretical investigation
... Pt), but they also include three other transition metals (Mn, Cr, and Ta). These conventional catalysts appear to have a moderate degree of reactivity toward carbon. If the reactivity is too strong, carbon is locked up in carbide instead of forming diamond. On the other hand, if the reactivity is to ...
... Pt), but they also include three other transition metals (Mn, Cr, and Ta). These conventional catalysts appear to have a moderate degree of reactivity toward carbon. If the reactivity is too strong, carbon is locked up in carbide instead of forming diamond. On the other hand, if the reactivity is to ...
Chapter 3: Minerals Why do we study minerals? They are the
... Physical properties of minerals The atomic structure evidenced in a number of physical properties. We will examine these links between atomic structure and hardness, density, cleavage, habit. Emphasize those properties underlined, as these are the most useful in identifying minerals. 1. Crystal habi ...
... Physical properties of minerals The atomic structure evidenced in a number of physical properties. We will examine these links between atomic structure and hardness, density, cleavage, habit. Emphasize those properties underlined, as these are the most useful in identifying minerals. 1. Crystal habi ...
Physical Properties of the Gemstones
... specific gravity. Heavier gemstones are usually harder as well. The range is from amber, which has a specific gravity of 1.08 and opal, with a specific gravity of 2.05, all the way up to corundum (sapphires and rubies) with a specific gravity of 3.99, spessartite garnet, specific gravity of 4.15, ma ...
... specific gravity. Heavier gemstones are usually harder as well. The range is from amber, which has a specific gravity of 1.08 and opal, with a specific gravity of 2.05, all the way up to corundum (sapphires and rubies) with a specific gravity of 3.99, spessartite garnet, specific gravity of 4.15, ma ...
Bonding + Physical Properties
... Diamond and graphite are both forms of carbon. Diamond is able to scratch almost all other substances, whereas graphite may be used as a lubricant. Diamond and graphite both have high melting points. Explain each of these properties of diamond and graphite in terms of structure and bonding. Give one ...
... Diamond and graphite are both forms of carbon. Diamond is able to scratch almost all other substances, whereas graphite may be used as a lubricant. Diamond and graphite both have high melting points. Explain each of these properties of diamond and graphite in terms of structure and bonding. Give one ...
Physical properties of minerals
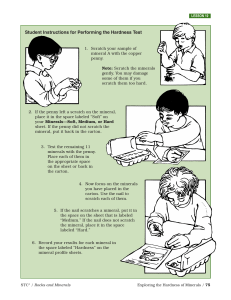
... • Hardness - This is the resistance of the mineral to abrasion or scratching. This property doesn't vary greatly from sample to sample of the same mineral, and thus is highly diagnostic. It also is a direct reflection of the bonding type and internal atomic arrangement. A value is obtained by compar ...
... • Hardness - This is the resistance of the mineral to abrasion or scratching. This property doesn't vary greatly from sample to sample of the same mineral, and thus is highly diagnostic. It also is a direct reflection of the bonding type and internal atomic arrangement. A value is obtained by compar ...
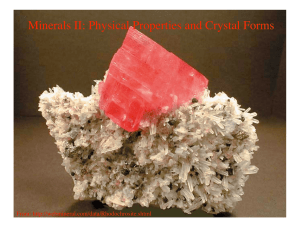
Minerals II: Physical Properties and Crystal Forms
... • Hardness - This is the resistance of the mineral to abrasion or scratching. This property doesn’t vary greatly from sample to sample of the same mineral, and thus is highly diagnostic. It also is a direct reflection of the bonding type and internal atomic arrangement. A value is obtained by compa ...
... • Hardness - This is the resistance of the mineral to abrasion or scratching. This property doesn’t vary greatly from sample to sample of the same mineral, and thus is highly diagnostic. It also is a direct reflection of the bonding type and internal atomic arrangement. A value is obtained by compa ...
MINERALS AND THEIR PROPERTIES
... 1: It is man-made (not naturally occurring) 2: It does not have a chemical composition because it is a mixture of iron and carbon 3: It does not have a crystal shape ...
... 1: It is man-made (not naturally occurring) 2: It does not have a chemical composition because it is a mixture of iron and carbon 3: It does not have a crystal shape ...
New Innovative Material The Kings School Robin HillsLonsdaleite
... Lonsdaleite is an allotrope of carbon, also called “hexagonal diamond”, because of the hexagonal lattice. It occurs naturally, forming when meteorites containing graphite hit the Earth. The graphite transforms into diamond due to the extreme heat and pressure of the impact. Scientists have also mana ...
... Lonsdaleite is an allotrope of carbon, also called “hexagonal diamond”, because of the hexagonal lattice. It occurs naturally, forming when meteorites containing graphite hit the Earth. The graphite transforms into diamond due to the extreme heat and pressure of the impact. Scientists have also mana ...
Preface - LegaLees
... come about in the past two centuries resulting from the developing science of geology and mineralogy and an increasing need to distinguish natural gemstones from those that are treated or grown in the laboratory. As a research scientist at the Gemological Institute of America (GIA) for more than 25 ...
... come about in the past two centuries resulting from the developing science of geology and mineralogy and an increasing need to distinguish natural gemstones from those that are treated or grown in the laboratory. As a research scientist at the Gemological Institute of America (GIA) for more than 25 ...
PdF-Article - Institut de Physique du Globe de Paris
... in which substitutional nitrogen impurities (i.e. N substituting for C atoms) occur in them. Type II diamonds have essentially no IR-active substitutional nitrogen, while type I diamonds contain > 10 ppm of substitutional nitrogen incorporated at the time of their formation. Type I diamonds are furt ...
... in which substitutional nitrogen impurities (i.e. N substituting for C atoms) occur in them. Type II diamonds have essentially no IR-active substitutional nitrogen, while type I diamonds contain > 10 ppm of substitutional nitrogen incorporated at the time of their formation. Type I diamonds are furt ...
Teacher Guide - Price9thScience
... generally white or clear in color, their ability to scatter light (“fire”) is superior to any other mineral. Diamonds are pure carbon, just like the soft graphite in a pencil. But while graphite is quite soft, diamonds owe their incredible hardness to the way that carbon atoms are arranged and bonde ...
... generally white or clear in color, their ability to scatter light (“fire”) is superior to any other mineral. Diamonds are pure carbon, just like the soft graphite in a pencil. But while graphite is quite soft, diamonds owe their incredible hardness to the way that carbon atoms are arranged and bonde ...
Argyle Diamond Mine – Geology and Mining of the AK1 Pipe
... samples of the Earth’s mantle roots of continents from depth of about 30 km to 200 km. Adjacent to diamond isotope ratios of osmium and other rare elements are of special interest, because they record processes over three billion years ago and clues to the composition of the Earth’s early mantle. Ec ...
... samples of the Earth’s mantle roots of continents from depth of about 30 km to 200 km. Adjacent to diamond isotope ratios of osmium and other rare elements are of special interest, because they record processes over three billion years ago and clues to the composition of the Earth’s early mantle. Ec ...
Minerals Elements and Minerals
... Rocks are classified into one of three families depending on how they formed. • Igneous, Metamorphic, Sedimentary ...
... Rocks are classified into one of three families depending on how they formed. • Igneous, Metamorphic, Sedimentary ...
Compound Minerals Reading Guide
... o What term is used to describe gemstones that are not genuine (real)? o The two common imitations are either made of 1. ___________________________________________________________ or 2. ___________________________________________________________ o Substitute gems may look beautiful, but they la ...
... o What term is used to describe gemstones that are not genuine (real)? o The two common imitations are either made of 1. ___________________________________________________________ or 2. ___________________________________________________________ o Substitute gems may look beautiful, but they la ...
10 - New Haven Science
... found near old riverbeds. How do you suppose the diamonds got there? Believe it or not, diamonds have even been found in meteorites from outer space! You probably know that diamonds are used in jewelry. But did you know they are also used in industry? In fact, out of every five diamonds found, only ...
... found near old riverbeds. How do you suppose the diamonds got there? Believe it or not, diamonds have even been found in meteorites from outer space! You probably know that diamonds are used in jewelry. But did you know they are also used in industry? In fact, out of every five diamonds found, only ...
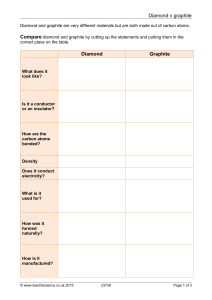
Diamond v graphite Diamond Graphite
... Diamond and graphite are very different materials but are both made out of carbon atoms. ...
... Diamond and graphite are very different materials but are both made out of carbon atoms. ...
Diamond – The April Birthstone
... Colors – the Gemological Institute of America (GIA) classifies low saturation yellow and brown diamonds as diamonds in the normal color range, and applies a grading scale from "D" (colorless) to "Z" (light yellow). Diamonds of a different color are called fancy colored diamonds and fall under a diff ...
... Colors – the Gemological Institute of America (GIA) classifies low saturation yellow and brown diamonds as diamonds in the normal color range, and applies a grading scale from "D" (colorless) to "Z" (light yellow). Diamonds of a different color are called fancy colored diamonds and fall under a diff ...
Material properties of diamond

Diamond is the allotrope of carbon in which the carbon atoms are arranged in the specific type of cubic lattice called diamond cubic. Diamond is an optically isotropic crystal that is transparent to opaque. Owing to its strong covalent bonding, diamond is the hardest naturally occurring material known. Yet, due to important structural weaknesses, diamond's toughness is only fair to good. The precise tensile strength of diamond is unknown, however strength up to 60 GPa has been observed, and it could be as high as 90–225 GPa depending on the crystal orientation. The anisotropy of diamond hardness is carefully considered during diamond cutting. Diamond has a high refractive index (2.417) and moderate dispersion (0.044) properties which give cut diamonds their brilliance. Scientists classify diamonds into four main types according to the nature of crystallographic defects present. Trace impurities substitutionally replacing carbon atoms in a diamond's crystal lattice, and in some cases structural defects, are responsible for the wide range of colors seen in diamond. Most diamonds are electrical insulators but extremely efficient thermal conductors. Unlike many other minerals, the specific gravity of diamond crystals (3.52) has rather small variation from diamond to diamond.