* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Atomic Mass - MrKanesSciencePage
Survey
Document related concepts
Transcript
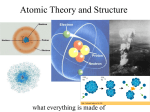
4-3 Atomic Structure Elements Contain a Single Kind of Atom • Robert Brown– Discovered direct evidence of the existence of atoms (1827). – His discovery is named after him. – Brownian Motion – perpetual jiggling of particles resulting from the collisions between visible particles (ie: dust) and invisible atoms. Brownian Motion in action More Brownian Motion In Action Elements Contain a Single Kind of Atom • Protons, Neutrons, and Electrons (subatomic particles) – Nucleus – The center of the atom, contains almost all of the mass of the atom. Contains both neutrons and protons. – Electron Cloud – Region outside the nucleus where the electrons travel. Sometimes referred to as electron shells. Typical model of a Helium atom In this model, we see the very small nucleus surrounded by a “cloud” of electrons whose shading relates to the likelihood of finding an electron at a given point at a given time. Atoms are Mostly Empty Space • Atoms contain mostly empty space due to their volumetrically large electron clouds. • Matter doesn’t “overlap” because rather than coming into complete contact, the +/repulsions prevent this, leaving an infinitely small gap between two objects moving toward one another. The Nucleus is Made of Protons and Neutrons • Proton (p+) – Found in the atomic nucleus – Carries a positive charge (1+) – About 2000 times more massive than an electron. • Neutron (no) – Found in the nucleus – Carries a neutral charge (0) – About the same mass as a proton. • Nucleons- a term that represents the protons and neutrons in the nucleus The Nucleus is Made of Protons and Neutrons • The number of protons in any atom of the same element will be the same, however, the number of neutrons can vary from atom to atom – This means that the number of protons in an atom determine the identity of that atom. • The number of protons (positive charge) equals the number of electrons (negative charge) in an atom. (All atoms are neutral in charge) The Nucleus is Made of Protons and Neutrons • Table of subatomic particles: Particle Charge Mass (in relationship to an electron Amu (Atomic Mass Unit) Proton (p+) 1+ 1,836 times the mass of an electron 1 Electron (e-) 1- 1 0 Neutron (no) 0 1841 1 Protons and Neutrons Determine the Mass Number and Atomic Mass • Atomic Mass - The average mass of all the different isotopes of an element. (Usually not a whole number) • Mass Number – The sum of the number of protons and neutrons. It is usually equal to the rounded atomic mass and is always expressed as a whole number. Protons and Neutrons Determine the Mass Number and Atomic Mass • Things to remember: – Mass number = # or protons + # of neutrons – Atomic number = # of protons – # of protons = # of electrons (for neutral atoms) Protons and Neutrons Determine the Mass Number and Atomic Mass • Isotope notation – a way to express the number of protons and mass number of a specific atom of an element. • Example: Lithium Mass # 7 Li Atomic # 3 Protons and Neutrons Determine the Mass Number and Atomic Mass • Chlorine Mass # 35 Cl Atomic # 17 Now You Try • Osmium 190 76 Os