* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 3: The Atom
Survey
Document related concepts
Transcript
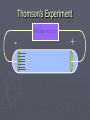
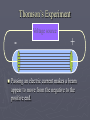
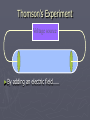
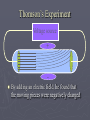
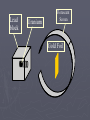
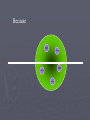
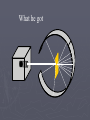
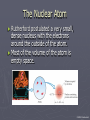
Chapter 3: The Atom “The Building Blocks of Matter” Early Definitions of the Atom ►The Greek philosopher Democritus (460 B.C. – 370 B.C.) was among the first to suggest the existence of atoms (from the Greek word “atomos”) He believed that atoms were indivisible and indestructible His ideas did agree with later scientific theory, but did not explain chemical behavior, and was not based on the scientific method – but just philosophy Aristotle ► Aristotle believed in 4 elements: ► Earth, Air, Fire, and Water. European Alchemists ► 1100 A.D. ► Their work evolved into what is now modern chemistry. ► Trying to change ordinary materials into gold. The 1700’s ► Little work was done on atomic theory until the 1700’s ► Aristotle’s and Democritus’ theories stood ► However some Laws were attained: Law of Conservation of Mass Law of Definite Proportions Law of Multiple Proportions Law of Conservation of Mass ► 1789: by French Chemist Antoine Lavoisier ► Mass is neither created nor destroyed during ordinary chemical reactions or physical changes ► Simply, the mass of what you end up with (products), must equal the mass of what you started with (reactants) Law of Definite Proportions ► 1800: French chemist Joseph Proust ► Chemical compounds contain the same elements in exactly the same proportions by mass, regardless of t he size of the sample ► Ex.) Salt, NaCl: always consists of 39.34% Sodium 60.66% Chlorine Law of Multiple Proproportions ► John Dalton first expressed this observation in 1803 and it is sometimes called Dalton's Law ► When elements combine they do so in a ratio of small whole numbers. Ex.) carbon and oxygen react to form CO or CO2, but not CO1.8 Video About Dalton Dalton’s Atomic Theory (experiment based!) John Dalton (1766 – 1844) 1) All matter is composed of extremely small particles called atoms. 2) Atoms of the same element are identical. Atoms of any one element are different from those of any other element. 3) Atoms cannot be subdivided, created, or destroyed. 4) Atoms of different elements combine in simple whole-number ratios to form chemical compounds Dalton Continued… 5) In chemical reactions, atoms are combined, separated, or rearranged – but never changed into atoms of another element. Experiments determining the structure of the atom ► Atom: smallest particle of an element that retains the chemical properties of that element. ► 1897: J. J. Thomson- used Cathode ray tubes Thomson’s Experiment Voltage source - + Thomson’s Experiment Voltage source - + Thomson’s Experiment Voltage source + Passing an electric current makes a beam appear to move from the negative to the positive end. Thomson’s Experiment Voltage source ► By adding an electric field…… Thomson’s Experiment Voltage source + By adding an electric field, he found that the moving pieces were negatively charged 1904: Thompson’s Model ► These were concluded to be electrons. ► Stability suggested that were there is a negative, there should be a positive. He couldn’t find the positive (for a while). ► Described the atom as plum pudding. ► A cloud of positive stuff, with the electrons able to be removed. 1909: Millikan’s Experiment Atomizer Oil spray + - Oil Microscope Millikan’s Experiment + _ X-rays X-rays charge the oil drops. Millikan’s Experiment Some drops would fall slower than others… From the mass of the drop and the charges on the two plates, he calculated the mass of and charge magnitude on an electron 1 electron = 9.11 x 10 –31 kg 1911: Earnest Rutherford’s Experiment ► Used uranium which as it decays produces alpha particles. ► Aimed alpha particles at gold foil by drilling hole in a lead block. ► Since the mass is evenly distributed in gold atoms, and if Thompson was correct in his structural picture of the atom, Rutherford believed the alpha particles should go straight through the foil. ► Used gold foil because it could be made very thin (only a few atoms thick). Lead block Uranium Florescent Screen Gold Foil What he expected Because Because, he thought the mass was evenly distributed in the atom. What he got Video About Rutherford How he explained it ► Atom is mostly empty ► Small dense, positive piece at center. ► Alpha particles (have a +2 charge) are deflected by it if they get close + enough. So Rutherford discovered the NUCLEUS!! + Modern View ► The atom is mostly empty space. ► Two regions ► Nucleus- protons and neutrons. ► Electron cloud- region where you might find an electron. ► More on this later… STOP Discovery of the Nucleus Ernest Rutherford shot particles at a thin sheet of gold foil and observed the pattern of scatter of the particles. © 2009, Prentice-Hall, The Nuclear Atom Since some particles were deflected at large angles, Thompson’s model could not be correct. © 2009, Prentice-Hall, Inc. The Nuclear Atom ► Rutherford postulated a very small, dense nucleus with the electrons around the outside of the atom. ► Most of the volume of the atom is empty space. © 2009, Prentice-Hall, Other Subatomic Particles ► Protons were discovered by Rutherford in 1919. ► Neutrons were discovered by James Chadwick in 1932. © 2009, Prentice-Hall, Inc. Subatomic Particles ► Protons and electrons are the only particles that have a charge. ► Protons and neutrons have essentially the same mass. ► The mass of an electron is so small we ignore it. © 2009, Prentice-Hall, Symbols of Elements Elements are symbolized by one or two letters. © 2009, Prentice-Hall, Atomic Number All atoms of the same element have the same number of protons: The atomic number (Z) © 2009, Prentice-Hall, Atomic Mass The mass of an atom in atomic mass units (amu) is the total number of protons and neutrons in the atom. © 2009, Prentice-Hall, Isotopes ► Isotopes are atoms of the same element with different masses. ► Isotopes have different numbers of neutrons. 11 C 6 12 C 6 13 C 6 14 C 6 © 2009, Prentice-Hall, Inc. Review Video Assignment ► Project 1: Atomic Structure Timeline Atomic Structure Timeline Requirements: ► Must include a title. ► Must include a spot for each of the 11 items. ► Must include at least the Who, When, and What. ► Must have a picture (color is preferred) for each item. Options: 1. Comic book format 2. Standard timeline 11 Important Figures/Discoveries ► Democritus ► Aristotle ► Antoine Lavoisier – Law ► Joseph Proust – Law ► John Dalton – Law ► John Dalton – Theory ► J.J. Thomson ► Robert Millikan ► Ernest Rutherford – Gold Foil ► Rutherford – Discovery of proton ► Chadwick – Discovery of neutron