* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Defining the Atom
Survey
Document related concepts
Transcript
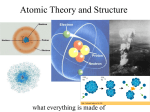
2.2 Defining the Atom > Early Models of the Atom II. The Atom: smallest particle of an element that retains its identity in a chemical reaction. a. Proposed first by Democritus who said atoms were indivisible and indestructible (400 BC) b. Dalton (1800): atoms of any one element are different from those of any other; atoms of the same element are identical; and different atoms can form mixtures or can chemically combine in simple whole-number ratios to form compounds. Slide 1 of 18 © Copyright Pearson Prentice Hall 2.2 Defining the Atom > Sizing up the Atom Iron Atoms Seen Through a Scanning Tunneling Microscope Slide 2 of 18 © Copyright Pearson Prentice Hall 2.2 Defining the Atom Subatomic Particles > A. Subatomic Particles: there are three 1. Protons: positively charged, in the nucleus a. Discovered1886, by Eugen Goldstein b. Large mass 2. Neutrons: no charge, in the nucleus a. Discovered 1932, by James Chadwick b. Mass nearly equal to that of a proton. 3. Electrons: are negatively charged a. J. J. Thomson discovered in 1897 b. Mass of proton / 1840 © Copyright Pearson Prentice Hall Slide 3 of 18 4.2 Defining the Atom > Subatomic Particles 5. Summarizes the properties (copy table) Slide 4 of 18 © Copyright Pearson Prentice Hall 4.2 Defining the Atom > The Atomic Nucleus 6. Shape of the Atom: a. Thompson said the atom was filled with positively charged material and the electrons were particles evenly distributed. b. Ernest Rutherford (1871–1937) directed a narrow beam of alpha particles at a very thin sheet of gold foil & found almost all of the mass concentrated in a small volume, the nucleus. i. atoms mostly low density space (electrons) c. nucleus: tiny central core, composed of protons and neutrons. © Copyright Pearson Prentice Hall Slide 5 of 18 4.2 Defining the Atom > The Atomic Nucleus d. electrons are distributed around the nucleus and occupy almost all the volume of the atom. Slide 6 of 18 © Copyright Pearson Prentice Hall 2.2 Defining the Atom Atomic Number > B. Distinguishing Among Atoms a. Different elements contain different numbers of protons. 1. Atomic Number: number of protons in the nucleus & the number of electron in a neutral atom. a. Periodic table arrange by this number. 2. Mass Number: total number of protons and neutrons. a. Slide 7 of 18 © Copyright Pearson Prentice Hall 4.3 Defining the Atom > Atomic Number Slide 8 of 18 © Copyright Pearson Prentice Hall 2.2 Defining the Atom > Isotopes 3. Isotopes: atoms that have the same number of protons but different numbers of neutrons and mass numbers. a. Chemically alike because of identical numbers of protons and electrons. Slide 9 of 18 © Copyright Pearson Prentice Hall 4.3 Defining the Atom > Atomic Mass 4. Atomic Mass: weighted average mass of the atoms in a naturally occurring sample of the element. a. Reflects both the mass and the relative abundance of the isotopes. b. atomic mass unit (amu) is defined as one twelfth of the mass of a carbon-12 atom. c. Example: Calculating atomic mass Slide 10 of 18 © Copyright Pearson Prentice Hall 4.3 Defining the Atom > Atomic Mass Weighted Average Mass of a Chlorine Atom 3 of 4 Cl atoms are Cl-35 1 of 4 Cl atoms are Cl-37 Slide 11 of 18 © Copyright Pearson Prentice Hall Defining the Atom Physical Science: PROFICIENCY PRACTICE > 119 P.12.A.2 http://www.rpdp.net/sciencetips_v2/P12A2.htm 119. The figure to the right shows part of the periodic table. Which of the following is an accurate comparison of the atomic number and mass of copper (Cu) and gold (Au)? A. Au has a smaller atomic mass and fewer electrons than Cu. B. Au has the same atomic mass as Cu but a greater atomic number. C. Au has the same atomic number as Cu but a much greater atomic mass. / D. Au has both a greater atomic number and a greater atomic mass than Cu. © Copyright Pearson Prentice Hall 29 Copper Cu 63.55 47 Silver Ag 107.9 79 Gold Au 197.0 Slide 12 of 18 Defining the Atom Physical Science : PROFICIENCY PRACTICE > 123 P.12.A.8 http://www.rpdp.net/sciencetips_v2/P12A8.htm 123. The mass number of an element is the total number of protons and neutrons located in the nucleus. If carbon has an atomic number of 6 and a mass number of 12, how many neutrons does it contain? A. 0 B. 6 C. 12 D. 18 Slide 13 of 18 © Copyright Pearson Prentice Hall Defining the Atom > 4.1 Slide 14 of 18 © Copyright Pearson Prentice Hall 4.1 Section Quiz 1. The ancient Greek philosopher credited with suggesting all matter is made of indivisible atoms is a. Plato. b. Aristotle. c. Democritus. d. Socrates. Slide 15 of 18 © Copyright Pearson Prentice Hall 4.2 Section Quiz 5. The nucleus of an atom consists of a. electrons only. b. protons only. c. protons and neutrons. d. electrons and neutrons. Slide 16 of 18 © Copyright Pearson Prentice Hall 4.2 Section Quiz 6. Most of the volume of the atom is occupied by the a. electrons. b. neutrons. c. protons and neutrons. d. protons. Slide 17 of 18 © Copyright Pearson Prentice Hall 4.3 Section Quiz 7. Isotopes of an element have a. the same mass number. b. different atomic numbers. c. the same number of protons but different numbers of neutrons. d. the same number of protons but different numbers of electrons. Slide 18 of 18 © Copyright Pearson Prentice Hall 4.3 Section Quiz 8. How many neutrons are in sulfur-33? a. 16 neutrons b. 33 neutrons c. 17 neutrons d. 32.06 neutrons Slide 19 of 18 © Copyright Pearson Prentice Hall