* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download History of the Atomic Theory
Survey
Document related concepts
Transcript
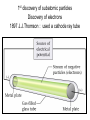
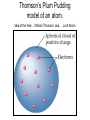
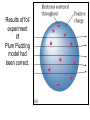
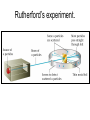
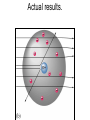
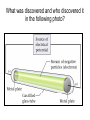
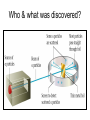
The student will learn: 1. 2. 3. 4. Development of modern day Atomic theory Early contributions of Democritus/Dalton Discovery of electron: Thompson/Millikan Discovery of nucleus: Rutherford/Chadwick History of the Atomic Theory 460-370 BC Democritus: small invisible particles called atomos. How? • 1766-1844 John Dalton Englishman Dalton’s Atomic theory • 1. element made up tiny particles called atoms • 2. atoms of a given element are identical • Ex) all carbon atoms have same physical & chemical properties atoms of a different element are different in some way Ex) carbon atoms have different physical & chemical properties than sulfur Dalton’s Atomic Theory • 3. Atoms of one element combine with atoms of a different element to form Compounds. * Law of Definite Proportion » All samples of a compound contain the same ratio / or / proportion of the elements * Chemical Formulas Dalton’s Atomic Theory 4.Chemical reactions involve reorganization of atoms * atoms are not changed just rearranged * atoms of one element cannot change into atoms of another element. “ cannot change copper into gold” Dalton’s Atomic Theory 1766-1844 1st discovery of subatomic particles Discovery of electrons 1897 J.J.Thomson : used a cathode ray tube Discovery of electrons Caused a vacuum in tube Filled it with a gas A piece of metal at each end + / Put electricity through it Surface opposite the cathode+ glowed Put magnets next to it = caused a deflection Placed an object inside it cast a shadow Placed a wheel inside and it moved Thomson’s Plum Pudding model of an atom. Idea of the time….William Thomson aka…. Lord Kelvin JJ Thomson 1897 Robert A. Millikan American physicist- 1909 Charge of electron 1.592 x 10-19 coulomb Oil drop experiment: suspended an oil drop between two charged plates. If made of pieces…….. then should be able to break it into pieces…….. Discovery of Nucleus Ernst Rutherford - 1911 Gold foil experiment: Bombarded a piece of gold foil with alpha particles which were considered positive. Expected to go all the way through it. Results of foil experiment if Plum Pudding model had been correct. Rutherford’s experiment. Actual results. p.72 “as if you had fired a 15 inch artillery shell at a piece of tissue paper and it bounced back”. Ernst Rutherford Thought about for 2 years. Rebounded positive 2He atom means Gold must have a dense middle and be positive. Named it nucleus and it must be positive. Ernest Rutherford 1911 James Chadwick discovery of neutron • In 1913 he was in Germany at start of World War I and would be interned in Ruhleben P.O.W. camp just outside of Berlin. • Hans Geiger and Ernest Rutherford interceded for his release. • It was his discovery of neutrons that led to “nuclear fission”…….sleeping pills Sir James Chadwick English - 1913 • Atoms from Dalton 1800 • Electrons from J.J.Thomson 1897 • Nucleus from Rutherford 1911 • Neutron from Chadwick 1932 • Protons???? • Collaborative but assigned to Rutherford….because needed something positive to make neutral. What was discovered and who discovered it in the following photo? Who & what was discovered?