* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download History of Atomic Theory
Survey
Document related concepts
Transcript
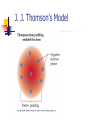
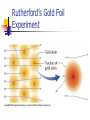
Atomic Theory Label the 3 different particles that make up an atom What is the charge of each? Draw a model of the element Boron (B) Development of atomic theory Democritus Atoms are indivisible, indestructible, fundamental units of matter. “Atomos” Never went further than this Aristotle Four elements of air, earth, water and fire. Regardless of the number of times you cut a form of matter, you would always have a smaller piece of that matter. Dalton’s Atomic Theory 1. All matter is composed of extremely small particles called atoms. 2. Atoms of given elements are identical in size, mass, and other properties. Dalton’s Atomic Theory 3. Atoms can not be subdivided, created, or destroyed. 4. Atoms of different elements can combine in simple, whole-number ratios to form chemical compounds. Dalton’s Atomic Theory 5. In a chemical reaction, atoms are combined, separated, or rearranged. Example: H2 + O2 H20 William Crookes Discovered cathode rays Set the stage for JJ Thompson and his discoveries J. J. Thomson Discovery of the electron Cathode ray tube Positively charged ions, called cations, move towards the cathode Negatively charged ions, called anions, move towards the anode. J. J. Thomson’s Model Robert Millikan oil drop experiment - calculated the mass of the electron Ernest Rutherford Existence of the nucleus and its relative size Gold foil experiment Rutherford’s Gold Foil Experiment Rutherford’s Gold Foil Experiment James Chadwick Discovered the last known subatomic particle The neutron which has no charge No. Not this Neutron… This neutron.