* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Unit 1 Matter review
Survey
Document related concepts
Transcript
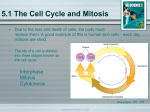
1.2 Investigating Matter • Matter is anything that has mass and volume. • Mass is the amount of matter in a substance or object. • Mass is often measured in grams or kilograms. • Volume is the amount of space a substance or an object occupies. • Volume is often measured in litres. • More information and practice on pages 480 - 483 See page 16 (c) McGraw Hill Ryerson 2007 1.3 Atomic Theory • Early ideas about matter • Greek philosophers believed that matter was made of atomos that were the smallest pieces of matter. • Aristotle believed matter was made of different combinations of earth, air, fire, and water. • Alchemists experimented with matter and tried to turn common metals into gold. Their activities marked the beginning of our understanding of matter. See pages 28 - 29 (c) McGraw Hill Ryerson 2007 Development of Atomic Theory I • John Dalton (1766 - 1844) • Credited with developing a theory that was a new way of explaining matter. • He studied gases that make up Earth’s atmosphere. Based on his studies, he suggested that: • matter is made of small, hard spheres that are different for different elements • the smallest particle of an element is called an atom • This is the basis for Dalton’s Atomic Theory. See page 29 (c) McGraw Hill Ryerson 2007 Dalton’s Atomic Theory 1. All matter is made of small particles called atoms. 2. Atoms cannot be created, destroyed, or divided into smaller particles. 3. All atoms of the same element are identical in mass and size, but they are different in mass and size from the atoms of other elements. 4. Compounds are created when atoms of different elements link together in definite proportions. See page 30 (c) McGraw Hill Ryerson 2007 Atomic Theory II • J. J. Thomson (1856 - 1940) • Thomson studied electric currents in gas discharge tubes (like today’s fluorescent lights). From his studies, he determined that the currents were streams of negatively charged particles. These were later called electrons. • He hypothesized that atoms are made of smaller particles. He proposed the “raisin bun” model of the atom. • This model is best visualized as a positively charged bun with negatively charge particles spread out in it like raisins. See page 30 (c) McGraw Hill Ryerson 2007 Atomic Theory III • Ernest Rutherford (1871 - 1937) • After experimenting with charged particles, he found that some particles were deflected in directions not originally predicted. • He suggested that the deflection of the charged particles was because the atom contained a tiny dense centre called a nucleus, and electrons moved around the nucleus. See page 31 (c) McGraw Hill Ryerson 2007 Atomic Theory IV • Niels Bohr (1885 - 1962) • He studied gaseous samples of atoms, which were made to glow by passing an electric current through them. • Based on his observations, Bohr proposed that electrons surround the nucleus in specific “energy levels” or “shells.” See page 31 - 32 (c) McGraw Hill Ryerson 2007 Inside the Atom • An atom is the smallest particle of an element that retains the properties of the element. • All atoms are made up of three kinds of particles called subatomic particles. These particles are: • electrons • protons • neutrons Take the Section 1.3 Quiz (c) McGraw Hill Ryerson 2007 See pages 32 - 33 Chemical Change • A chemical change is a change in matter that occurs when substances combine to form new substances. • For example, fireworks • Find Out Activity 1-2A Bag of Change See page 17 (c) McGraw Hill Ryerson 2007 Physical Change and Changes of State • When a physical change occurs, there may be a change in appearance, but no new substances are formed. • For example, when ice or snow melts to water, this physical change is a change of state. No new substances are formed. See page 18 (c) McGraw Hill Ryerson 2007 The Particle Model of Matter • Describes the behaviour of matter • Matter is made of small particles. • There are spaces between the particles. • Gases have more space than liquids. Liquids have more space than solids. • Particles are always moving. • Particles are attracted to each other. The strength of attraction depends on the type of particle. See page 18 (c) McGraw Hill Ryerson 2007 The Kinetic Molecular Theory • Describes what happens to matter when the kinetic energy of particles changes. The main points in the theory are: • Matter is made of small particles. • There is empty space between particles. • Particles are constantly moving. • Solid particles are packed together and cannot move freely. They can only vibrate. • Liquid particles are farther apart and can slide past each other. • Gas particles are far apart and move around quickly. • Energy makes particles move. See page 19 (c) McGraw Hill Ryerson 2007 The Kinetic Molecular Theory and Changes of State Solid Particles are close together, fixed in position and vibrating. Melting As temperature increases, particles’ kinetic energy increases. Liquid Particles are still close, but slide past one another. Boiling As temperature increases, particles’ kinetic energy continues to increase, creating more space. Gas Particles are highly energetic and moving freely. See page 20 (c) McGraw Hill Ryerson 2007 Temperature and Changes of State See page 21 (c) McGraw Hill Ryerson 2007 Describing Matter • Physical Properties • Qualitative - state, colour, malleability • Quantitative - conductivity, viscosity, density • Pure Substances • Element - a pure substance that cannot be broken down or separated into simpler substances (e.g., gold) • Compound - a pure substance composed of at least two elements (e.g., water) Take the Section 1.2 Quiz (c) McGraw Hill Ryerson 2007 See pages 22 - 23 2.1 Elements • Why are elements studied in chemistry? • Chemistry is the study of matter and its changes. • Elements make up an incredible variety of different substances. • An element is a pure substance that cannot be broken down or separated into simpler substances. Each element is one kind of atom. • By studying elements, we can learn more about the structure of matter. See page 42 (c) McGraw Hill Ryerson 2007 Chemical Symbols • Element names and symbols • Because elements have different names in different languages, chemists use international symbols for them • Chemical symbols consist of one or two letters. • Ancient names are used as the source of many of the symbols. Example: • Mercury - Hg - Hydragyrum (Latin for liquid silver) See pages 43 - 44 (c) McGraw Hill Ryerson 2007 Chemical Symbols All elements are represented by symbols. Here are just a few element symbol examples: See page 44 (c) McGraw Hill Ryerson 2007 Common Elements • Hydrogen • Colourless, odourless, tasteless, and highly flammable gas. • Makes up over 90 percent of the atoms in the universe • Used in producing fertilizers • Lighter than air • Can be separated from water or gasoline and be used as a source of fuel See page 45 (c) McGraw Hill Ryerson 2007 Common Elements • Iron (Fe) - mixed with carbon to make steel • Good structural material, but can rust when mixed with water or oxygen • Oxygen (O) - gaseous element we breathe • 21 % of the atmosphere • Reacts with most other elements Oxygen and iron react in burning thermite GNU license photo Iron in a river turns water and rocks red See page 45 (c) McGraw Hill Ryerson 2007 Other Common Elements • • • • • Sodium (Na) - soft metal that reacts with water Chlorine (Cl) - yellow-green gas that is highly toxic Mercury (Hg) - liquid at room temperature metal. Silver (Ag) - precious metal mined in British Columbia Silicon (Si) - brittle, grey, semiconductor that is second most common element in Earth’s crust. Na Cl Hg Ag Take the Section 2.1 Quiz (c) McGraw Hill Ryerson 2007 Si See pages 46 - 47 2.2 Periodic Table • Origin of the periodic table • Chemists in the 10th century wished to organize elements • Attempts focused on grouping elements with similar properties • In 1867, Dimitri Mendeleev found patterns in the elements and organized them into table • The resulting table had holes for elements not yet discovered See page 52 (c) McGraw Hill Ryerson 2007 Periodic Table • The Periodic Table provides information on the physical and chemical properties of elements Atomic Mass - mass of average atom Atomic Number - number of protons Ion Charge - electric charge that forms when an atom gains or loses electrons See page 53 (c) McGraw Hill Ryerson 2007 Periodic Table See page 54 (c) McGraw Hill Ryerson 2007 Metals, Non-metals, Metalloids • Period table has interesting patterns • Due to Mendeleev’s organization, interesting patterns are created, such as the groups: metals, non-metals and metalloids. See page 55 (c) McGraw Hill Ryerson 2007 Periods and Families • Each horizontal row in the periodic table is a period • Vertical columns form groups or chemical families • Alkali metals - highly reactive group 1 • Alkaline earth metals - group 2, burn in air if heated • Halogens - group 17, highly reactive non-metals • Noble gases - group 18, stable and unreactive non-metals Take the Section 2.2 Quiz (c) McGraw Hill Ryerson 2007 See pages 56 - 57 2.3 Periodic Table and Atomic Theory • Elements with similar properties have similar electron arrangements • Bohr models show electron arrangement in shells See page 64 - 65 (c) McGraw Hill Ryerson 2007 Bohr model patterns • Chemical families on the periodic table have the same number of valence electrons • Elements in the same period have the same number of shells • Period number indicates the number of electron shells See page 66 (c) McGraw Hill Ryerson 2007 Atom Stability • Noble gases are very unreactive because their atoms have filled valence shells. Filled shells make atoms stable. Atoms with filled shells do not easily trade or share electrons. • Other atoms gain or lose electrons in order to achieve the stability of noble gases. Gaining or losing electrons makes atoms into ions. • • • • Metals lose electrons to form positive ions Non-metals gain electrons to form negative ions Ions have a similar electron arrangement to the nearest noble gas Example: Sodium ion (Na+) has 11 protons (11+) and 10 electrons (10-) for a total charge of 1+ Take the Section 2.3 Quiz (c) McGraw Hill Ryerson 2007 See pages 66 - 67 3.1 Compounds • Compounds are pure substances made of more than one kind of atom joined together. The atoms are held together with chemical bonds. • Compounds come in two basic types: covalent and ionic. Covalent compounds share electrons to form molecules. Example: water In ionic compounds, atoms gain or lose electrons to form ions. Example: NaCl See pages 76 - 78 (c) McGraw Hill Ryerson 2007 Ionic Compounds • Ionic solids exist as a solid in the form of an ionic lattice. • The positive ions attract all of the negative ions, and vice versa. In the example of table salt (NaCl) the one-to-one ratio of ions results in a simple square-shaped ionic cyrstal: See page 78 (c) McGraw Hill Ryerson 2007 Polyatomic Ions • Covalent and ionic bonds can occur together • A molecule can gain or lose electrons to become charged, forming a polyatomic ion. • Polyatomic ions form compounds like other ions. • Example: Ammonium ion (NH4+) • There are many types of polyatomic ions, but they occur in a few basic shapes. Take the Section 3.1 Quiz (c) McGraw Hill Ryerson 2007 See pages 79 - 80 3.2 Names and Formulas of Ionic Compounds • The chemical name indicates the elements present in the compound. Chemical names for ionic compounds are given according to rules. • The positive ion is always the first part of the name • The negative ion is always the second part of the name • The non-metal ion’s name ends with the suffix “-ide” See page 85 (c) McGraw Hill Ryerson 2007 Ionic Chemical Formulas • In an ionic compound, the positive charges balance the negative charges. This balance of charge is used to determine the smallest whole number ratio of positive to negative ions. See page 87 (c) McGraw Hill Ryerson 2007 Multivalent Metal Compounds • Many metals are multivalent, meaning the metals form two or more different positive ions with different charges • For example, the atom iron forms two ions Fe2+ and Fe3+ • Too distinguish different ions for the same metal, roman numerals are added to their name. For example, Fe3+ would be named “iron(III)” See page 88 (c) McGraw Hill Ryerson 2007 Writing Multivalent Formulas • Writing ionic compound formulas with multivalent ions follows the same rules as regular ionic compounds See page 89 (c) McGraw Hill Ryerson 2007 Multivalent Compound Names • Steps to writing multivalent compound names are as follows: • Identify the metal and verify it forms more than one ion • Determine the ratio of ions - for example, Fe2O3 means 2 iron ions for every 3 oxygen ions • Note the charge on the negative ion: Oxygen is O2• The positive and negative charges must balance, so 2 iron ions of 3+ charge (Fe3+) are needed to balance the 3 oxygen ions • Write the name of the compound: Iron(III) oxide See page 90 (c) McGraw Hill Ryerson 2007 Polyatomic Ion Compounds • Steps to writing names for formulas involving polyatomic ions are similar to other ionic compounds Take the Section 3.2 Quiz (c) McGraw Hill Ryerson 2007 See page 91 3.3 Physical and Chemical Changes • • In physical changes, the appearance of a substance changes, but the chemical bonds holding the substance together do not change. Examples: melting, freezing, boiling In chemical changes, new substances are produced in the process of breaking chemical bonds and forming new ones. • Evidence of chemical change: • • • • • Colour change Heat, light, sound produced or consumed Bubbles of gas form Formation of a precipitate The change is difficult to reverse See page 98 (c) McGraw Hill Ryerson 2007 Energy Changes • In both physical and chemical changes, energy changes take place. This energy change can mean releasing to or absorbing energy from the environment. • Exothermic reactions involve the overall release of energy in the form of heat and light. • Endothermic reactions involve the overall absorption of energy. Instant Cold Pack: Endothermic Campfire: exothermic See page 99 (c) McGraw Hill Ryerson 2007 Applications of Chemical Changes • Some chemical changes present problems, while others provide opportunities and advantages • Corrosion is major problem for steel structures - by protecting steel surfaces, the chemical reaction of iron with oxygen can be prevented. • First nations people of the Pacific Coast have used smoking as a means of preserving food. Smoke causes chemical changes in meat that kill bacteria. Take the Section 3.3 Quiz (c) McGraw Hill Ryerson 2007 See page 100