* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download L 35 Modern Physics [1]
Hydrogen atom wikipedia , lookup
Delayed choice quantum eraser wikipedia , lookup
Particle in a box wikipedia , lookup
Atomic orbital wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Tight binding wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Double-slit experiment wikipedia , lookup
Matter wave wikipedia , lookup
Electron configuration wikipedia , lookup
Electron scattering wikipedia , lookup
Laser pumping wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Population inversion wikipedia , lookup
Atomic theory wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
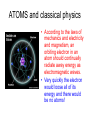
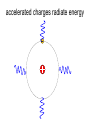
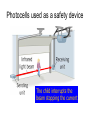
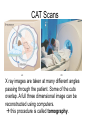
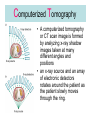
L 33 -Modern Physics • “Modern” – 20th Century • By the end of the 19th century it seemed that all the laws of physics were known • However, there were a few problems where classical physics (pre-20th century) didn’t seem to work. • It became obvious that Newton’s laws could not explain atomic level phenomena Newton’s Laws have flaws! • Newton’s laws, which were so successful in allowing us to understand the behavior of big objects such as the motions of the planets, failed when pushed to explain atomic size phenomena. • The discovery of the laws of atomic physics led to every important 20th century discovery that have transformed our lives, the electronic revolution. Newton’s laws also fail at high velocities • According to Newton’s laws, if we keep exerting a force on an object, it will continue to accelerate and there is no limit to how fast the particle can go • According to Einstein, nothing can be accelerated to a speed greater than the speed of light, 186,000 miles per second ATOMS and classical physics • According to the laws of mechanics and electricity and magnetism, an orbiting electron in an atom should continually radiate away energy as electromagnetic waves. • Very quickly the electron would loose all of its energy and there would be no atoms! accelerated charges radiate energy The photoelectric effect – defies a classical explanation! LIGHT photoelectrons Metal plate • When light shines on a metal surface, electrons pop out – we call these photoelectrons • Photoelectrons are only emitted if short wavelength light (blue) is shined on the metal, no matter how intense the light is. Details of a photocell Photocells used as a safety device The child interrupts the beam stopping the current Einstein explains the PE effect, receives Nobel Prize in 1921 • Light is an electromagnetic wave, but when it interacts the metal it behaves like a particle, a light particle called a photon. • A beam of light is a beam of photons. number Photon Energy = Wavelength • Blue photons are more energetic than red photons, so blue photons can knock electrons out of the metal The quantum concept • The photon concept is a radical departure from classical thinking. • In classical physics, energy can come in any amounts • In modern physics, energy is QUANTIZED comes in definite packets photons • In the PE effect energy is absorbed by the electrons only in discreet amounts Video recorders and digital cameras A CCD (charge coupled device) can be used to capture photographic Images using the photoelectric effect. http://money.howstuffworks.com/camcorder2.htm The quantum concept and the Bohr Atom • Niels Bohr, a Danish physicist, used the quantum concept to explain the nature of the atom. • Recall that the orbiting electrons, according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a continuous range of wavelengths (colors) NOT SO! The Bohr Atom Nucleus + Ei Ef The orbits farther from the nucleus are higher energy states than the closer ones • The electrons move in certain allowed, “stationary” orbits or states in which then do not radiate. • The electron in a high energy state can make a transition to a lower energy state by emitting a photon whose energy was the difference in energies of the two states, hf = Ei - Ef Line spectra of atomic hydrogen The Bohr model was successful in determining Where all the spectral lines of H should be. Line spectra of atoms Line spectra are like atomic fingerprints. Forensic scientists use line spectra to identify substances. THE LASER: a product of 20th Century Physics Light Amplification by Stimulated Emission of Radiation. Laser Type Wavelength (nm) Argon fluoride (UV) 193 Krypton fluoride (UV) 248 Xenon chloride (UV) 308 Nitrogen (UV) 337 Argon (blue) 488 Argon (green) 514 Helium neon (green) 543 Helium neon (red) 633 Rhodamine 6G dye (tunable) Ruby (CrAlO3) (red) Nd:Yag (NIR) Carbon dioxide (FIR) 570-650 694 1064 10600 What’s special about laser light? Laser Light • • • • • • stimulated light emission by atoms Monochromatic (one pure color) coherent – all the waves travel in step all waves travel in same direction waves reinforce each other applications – scanners and CD readers, CD burners – cut metals – laser surgery Medical Applications of Lasers Laser surgery to correct for (a) nearsightedness, and (b) farsightedness How does a CD burner Work? • infrared laser light is applied to a layer of photosensitive dye on top of the plastic • this causes the dye to darken (no burning!) • by selectively darkening particular points along the CD track, and leaving other areas of dye translucent, a digital pattern is created that can be read by a standard CD player http://computer.howstuffworks.com/cd-burner4.htm Laser Fusion Multiple beams of a powerful laser are focused on a tiny pellet containing fusion fuel. The laser energy compresses the pellet producing a mini-hydrogen bomb that produces energy Medical Imaging Techniques • x-rays • CT and CAT scans (Computerized Tomography) • MRI’s (Magnetic Resonance Imaging) X-rays x-ray of Homer’s head • very short wavelength (0.01 – 0.1 nm) electromagnetic waves • produced when energetic electrons slam into a metal target • able to penetrate soft tissue, but not bone • produces a two dimensional shadow image A pineapple and a banana • A shadow image can be misleading • two shadows taken from different angles provides a better picture • shadows taken at multiple angles gives a more complete picture • this is what a CT or CAT scan does CAT Scans X ray images are taken at many different angles passing through the patient. Some of the cuts overlap. A full three dimensional image can be reconstructed using computers. this procedure is called tomography. Computerized Tomography • A computerized tomography or CT scan image is formed by analyzing x-ray shadow images taken at many different angles and positions • an x-ray source and an array of electronic detectors rotates around the patient as the patient slowly moves through the ring.