* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The History of the Modern Periodic Table
Alkali metal wikipedia , lookup
Group 12 element wikipedia , lookup
Boron group wikipedia , lookup
Alkaline earth metal wikipedia , lookup
Group 3 element wikipedia , lookup
Period 6 element wikipedia , lookup
Period 3 element wikipedia , lookup
Period 5 element wikipedia , lookup
The History of
the Modern
Periodic Table
During the nineteenth century,
chemists began to categorize the
elements according to similarities
in their physical and chemical
properties. The end result of
these studies was our modern
periodic table.
John Newlands
In 1863, he suggested that elements be
arranged in “octaves” because he noticed
(after arranging the elements in order of
increasing atomic mass) that certain
properties repeated every 8th element.
Law of Octaves
1838 - 1898
John Newlands
Newlands' claim to see a repeating pattern was met
with savage ridicule on its announcement. His
classification of the elements, he was told, was as
arbitrary as putting them in alphabetical order and
his paper was rejected for publication by the
Chemical Society.
1838 - 1898
Law of Octaves
John Newlands
His law of octaves failed beyond the
WHY?
element calcium.
Would his law of octaves work today with
the first 20 elements?
1838 - 1898
Law of Octaves
Dmitri Mendeleev
In 1869 he published a table of
the elements organized by
increasing atomic mass.
1834 - 1907
Mendeleev’s discovery
The fateful day for Mendeleev was 17 February 1869 (Julian
calendar). He cancelled a planned visit to a factory and stayed
at home working on the problem of how to arrange the
chemical elements in a systematic way. To aid him in this
endeavor he wrote each element and its chief properties on a
separate card and began to lay these out in various patterns.
Mendeleev’s discovery
Eventually he achieved a layout that suited him and
copied it down on paper. Later that same day he
decided a better arrangement was possible and made a
copy of that, which had similar elements grouped in
vertical columns, unlike his first table, which grouped
them horizontally. These historic documents still exist.
That Mendeleev realized that he had discovered, rather
than designed, the periodic table is shown by his
attitude towards it.
Mendeleev’s discovery
First, he left gaps in it for missing elements. Leaving such
gaps in tables of elements was not in itself new, but
Mendeleev was so sure of himself that he was prepared to
predict the physical and chemical properties of these
undiscovered elements. His most notable successes were
with eka aluminium (= Gallium) and eka-silicon (=
germanium). Lecoq de Boisbaudran discovered gallium in
1875 and reported its density as 4.7g cm -3, which did not
agree with Mendeleev’s prediction of 5.9 g cm -3.
Mendeleev’s discovery
When he was told that his new element was Mendeleev’s
eka-aluminium, and had most of its properties foretold
accurately, Boisbaudran redetermined its density more
accurately and found it to be as predicted, 5.956 g cm -3.
There could be no doubt now that Mendeleev had discovered
a fundamental pattern of Nature.
Mendeleev’s discovery
Lothar Meyer
At the same time, he published his own
table of the elements organized by
increasing atomic mass.
1830 - 1895
Elements known at this time
Mendeleev...
• stated that if the atomic weight of an
element caused it to be placed in the
wrong group, then the weight must be
wrong. (He corrected the atomic
masses of Be, In, and U)
• was so confident in his table that he
used it to predict the physical
properties of three elements that were
yet unknown.
After the discovery of these unknown
elements between 1874 and 1885, and the
fact that Mendeleev’s predictions for Sc,
Ga, and Ge were amazingly close to the
actual values, his table was generally
accepted.
However, in spite of Mendeleev’s great
achievement, problems arose when new
elements were discovered and more
accurate atomic weights determined. By
looking at our modern periodic table, can
you identify what problems might have
caused chemists a headache?
Ar and K
Co and Ni
Te and I
Th and Pa
Henry Moseley
In 1913, through his work with X-rays, he
determined the actual nuclear charge
(atomic number) of the elements*. He
rearranged the elements in order of
increasing atomic number.
*“There is in the atom a fundamental
quantity which increases by regular
steps as we pass from each element to
the next. This quantity can only be the
charge on the central positive nucleus.”
1887 - 1915
Henry Moseley
His research was halted when the British
government sent him to serve as a foot
soldier in WWI.
He was killed in the
fighting in Gallipoli by a sniper’s bullet, at
the age of 28. Because of this loss, the
British government later restricted its
scientists to noncombatant duties during
WWII.
Periodic Table
Geography
The horizontal rows of the periodic table
are called PERIODS.
The elements in any group
of the periodic table have
similar physical and chemical
properties!
The vertical columns of the periodic table
are called GROUPS, or FAMILIES.
Periodic Law
When elements are arranged in order of
increasing atomic number, there is a
periodic pattern in their physical and
chemical properties.
Alkali Metals
Alkaline Earth Metals
Transition Metals
These elements are also
called the rare-earth
elements.
InnerTransition Metals
Halogens
Noble Gases
The s and p block elements
are called
REPRESENTATIVE ELEMENTS.
Modern Periodic Table
The “Real” Periodic Table
Modern Periodic Table
Groups
• Alkali Metals (Group 1)
– most reactive metals
– never found alone in nature
– lose 1 e- to achieve stability
• Brainiac Video
• Alkaline Earth Metals (Group 2)
– harder, denser, stronger, higher melting points
than Group 1
– less reactive than Group 1
– will lose 2 e- to achieve stability
• Transition Metals (Groups 3-12)
– metals with 1 or 2 e- in their outer energy level
– less reactive than Group 1 & 2
• some exist in nature as free elements (Au, Pd, Pt,
Ag)
– lose e- to achieve stability
Inner Transition Metals
• Lanthanides—elements 57-70
– all very similar to each other
– this is why they are not placed in a group
– all are metals, some are very strong magnets
• Actinides—elements 89-102
– all are radioactive
– most are synthetic
– all are metals
• CARBON
– can lose, gain, or share 4 e– Prefers to share. Why?
– Element of life
• Halogens (Group 17)
– most reactive nonmetals
– never found alone in nature
– gain or share e- to achieve stability
Chlorine
Bromine
Iodine
• Noble Gases (Group 18)
– Discovered between 1858 and 1900
– inert
– Newest Group
• HYDROGEN (H)
–
–
–
–
–
doesn’t belong to any group
unique properties
nonmetal
placed where it is based on e- config.
can lose, gain, or share its 1 e-
Metals, Nonmetals, & Metalloids
Metals
• hard, shiny (lustrous), good conductor, loses
e- to form compounds, usually 2 or less ein the outer energy level
• malleable – hammered
into sheets
• ductile – drawn into wires
Nonmetals
• gases or brittle solids
• insulators, gain or share e- to form compounds
• usually have 5 or more e- in outer energy level
Metalloids
• semiconductors
• properties of both metals and nonmetals
Electron Configurations
• An element’s chemical properties are
determined by its e- config.
• Differences in properties of elements are
due to different e- configurations, not just
placement on the chart.
Ions
• Ion – an atom or group of atoms having a charge
– can be (+) or (-)
– charged due to gain or loss of electrons
• Cation – positive ion, forms when metals LOSE e• Anion – negative ion, forms when nonmetals GAIN e-
Ions
Ions
Oxidation Number
• The Oxidation Number (charge) tells the number of electrons
an atom will lose or gain to achieve stability
Group 1
Group 2
Group 18
Group 17
Group 16
=
=
=
=
=
+1
+2
0
-1
-2
Aluminum
Zinc
Cadmium
Silver
Carbon
= +3
= +2
= +2
= +1
= -4 or +4
Group 15 = -3 (nonmetals)
Hydrogen
= +1 or -1
Transition metals can lose e- from “s” or “d” sublevel or both,
multiple oxidation #s (usually +1 or +2).
Periodic
Trends
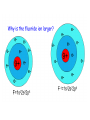
Atomic Radius
• Atomic Radius – how big an atom is (p. 163)
– decreases across a period b/c Zeff increases
– increases down a group b/c more shells are
added
Increase
Atomic Radius
I
n
c
r
e
a
s
e
One half the distance between
nuclei of identical atoms that
are bonded together.
Periodic Trends
• Ionic Radius – how big an ion is (charged particle);
– Metal ions – have lost electrons, charge is (+), cation
• Ionic radius is smaller than atomic radius
• isoelectronic with preceding noble gas
• size of metal cations
– decreases across a period
– increases down a group
– Nonmetal ions – have gained electrons, charge is (-), anion
• Ionic radius is larger than atomic radius
• isoelectronic with following noble gas
• size of nonmetal anions
– decreases across a period
– increases down a group
Ionic Radius
Ionic Radius
I
n
c
r
e
a
s
e
One half the distance between
nuclei of
Identical ions that are bonded
together.
Electronegativity
• Electronegativity – the ability of an atom to attract e- when in
a compound (p.168)
– Increases across a period. Why?
• Increased Zeff attracts electrons closer to nucleus.
– decreases down a group
Electronegativity
The measure of the ability
of an atom in a
chemical compound to
attract electrons
Ionization Energy
• Ionization Energy – energy required to remove an
e- from an atom
– (units = kJ/mol) {p167.}
– First Ionization Energy – energy required to remove
most loosely held e• increases across a period
• decreases down a group
• Metals have low ionization energies. Why?
– They easily lose electrons in order to become stable.
• Nonmetals have high ionization energies. Why?
– Losing electrons makes them more unstable.
Ionization Energy
The amount of energy
required to remove one
electron from a neutral atom
I
n
c
r
e
a
s
e
Shielding
• Trends affecting ionization energy
– Shielding – inner e- block Zeff attraction of (+)
nucleus for outer e•
•
•
•
constant across a period
increases down a group
e- feel less charge
How does this affect ionization energy?
– It causes IE to increase
Zeff
• Zeff = effective nuclear charge
• This is how much charge the negative valence
electrons “feel” from the positive protons
• Zeff can be approximated by subtracting the
number of inner (non-valence) electrons from
the atomic number.
*Fluorine's outer electrons experience a stronger
attraction to the nucleus and are pulled in closer.
The periodic table is the most important
tool in the chemist’s toolbox!