* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 4-3-Isotopes
Survey
Document related concepts
Transcript
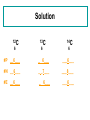
Warm Up O 8 p+ 8n 8 e- 2O 8 p+ 8n 10 e- 2+ Zn 30 p+ 35 n 28 e- Same Element Different AtomIsotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes) Learning Objectives You will know what isotopes are You will be able to figure out the number of neutrons for each isotope You will be able to calculate the atomic mass of an element that has different isotopes. Isotope Example 35Cl 37Cl 17 17 chlorine - 35 chlorine - 37 Isotopes of Carbon Naturally occurring carbon consists of three isotopes, 12C, 13C, and 14C. State the number of protons, neutrons, and electrons in each of these carbon atoms. 12C 6 13C 14C 6 6 #P _______ _______ _______ #N _______ _______ _______ #E _______ _______ _______ Solution 12C 6 13C 14C 6 6 #P __6___ _ 6___ ___6___ #N __6___ _ _7___ ___8___ #E __6___ _ 6___ ___6___ Zinc problem An atom of zinc has a mass number of 65. A. Number of protons in the zinc atom 1) 30 2) 35 3) 65 B. Number of neutrons in the zinc atom 1) 30 2) 35 3) 65 C. What is the mass number of a zinc isotope with 37 neutrons? 1) 37 2) 65 3) 67 Solution to Zinc Problem An atom of zinc has a mass number of 65. A. Number of protons in the zinc atom 1) 30 B. Number of neutrons in the zinc atom 2) 35 C. What is the mass number of a zinc isotope with 37 neutrons? 3) 67 Atomic Mass Listed on the periodic table Na 22.99 Gives the mass of “average” atom of each element compared to 12C Average atom based on all the isotopes and their abundance % Atomic mass is not a whole number … mass number is a whole number Calculating Atomic Mass Percent(%) abundance of isotopes Mass of each isotope of that element Weighted average = mass isotope1(%) + mass isotope2(%) + … 100 100 Atomic Mass of Magnesium Isotopes Mass of Isotope Abundance 24Mg = 24.0 amu 78.70% 25Mg = 25.0 amu 10.13% 26Mg = 26.0 amu 11.17% (24)(.787) + (25)(.1013) + 26(.1117) = 18.888 + 2.5325 + 2.9042 = 24.3 amu