* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download AEROSOL INDIRECT EFFECT
Climate governance wikipedia , lookup
Mitigation of global warming in Australia wikipedia , lookup
Climate change adaptation wikipedia , lookup
Climate engineering wikipedia , lookup
Climate change in Tuvalu wikipedia , lookup
Atmospheric model wikipedia , lookup
Media coverage of global warming wikipedia , lookup
Politics of global warming wikipedia , lookup
Climate sensitivity wikipedia , lookup
Effects of global warming on human health wikipedia , lookup
Citizens' Climate Lobby wikipedia , lookup
Scientific opinion on climate change wikipedia , lookup
Public opinion on global warming wikipedia , lookup
Climate change and agriculture wikipedia , lookup
Global warming wikipedia , lookup
General circulation model wikipedia , lookup
Surveys of scientists' views on climate change wikipedia , lookup
Effects of global warming on humans wikipedia , lookup
Climate change in the United States wikipedia , lookup
Effects of global warming on Australia wikipedia , lookup
Attribution of recent climate change wikipedia , lookup
Climate change and poverty wikipedia , lookup
Climate change, industry and society wikipedia , lookup
Years of Living Dangerously wikipedia , lookup
IPCC Fourth Assessment Report wikipedia , lookup
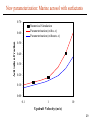
AEROSOL INDIRECT EFFECT: THE ELUSIVE COMPONENT OF CLIMATE CHANGE Athanasios Nenes HDGC Seminar, November 20, 2002 photo: G.Roberts Global warming vs. climate change. Global warming is only one aspect. We are really looking at is climate change. Air pollution is much more than greenhouse gases. Aerosols (suspended particles) are a major component: why? 2 Research focus: climate change. Aerosols and their interaction with clouds play a major role in the climate system. J.T. Houghton: “The science of climate change” 3 Why are we interested in aerosols? Aerosols are emitted together with greenhouse gases. Pollution plume 1500 km south of India. Outside of the plume. Pristine state. 4 Why are we interested in aerosols? Pollution plumes off of Asia: they are of continental proportions. 5 Why are we interested in aerosols? Biomass burning in the Amazon. 6 Research focus: aerosol effects on clouds. Clouds play a major role in the climate system. A small change in cloud properties can strongly affect climate. 7 Question: How do clouds form? Answer: from aerosols. Clouds form in regions of the atmosphere where water vapor is supersaturated. We focus on liquid water clouds. Water vapor supersaturation is generated by cooling (primarily through expansion in updraft regions and radiative cooling) Cloud droplets form from pre-existing particles found in the atmosphere (aerosols). This process is known as activation. Aerosols that can become droplets are called cloud condensation nuclei (CCN). Cloud Aerosol particle that does not activate CCN that activates into a cloud drop 8 Example of cloud droplet formation in an updraft. log10(concentration) movie drop growth activation aerosol log10(size) 9 What is the aerosol indirect effect? It is the change in cloud properties caused by a change in the CCN population. Cloud properties are a strong function of droplet concentration. Two kinds of indirect effects lead to climatic cooling : • Increase in cloud reflectivity • Increase in cloud lifetime & coverage. Less polluted More polluted Smaller droplets: clouds reflect more and last longer 10 Observational evidence of indirect effect “Ship tracks”: linear features of high cloud reflectivity embedded in marine stratus clouds, resulting from aerosols emitted by ships. M.Kulmala: “Nucleation and Atmospheric Aerosols, 1996” 11 Anthropogenic indirect forcing: least understood. • Potentially of large magnitude. (comparable to greenhouse gas warming) • Cooling effect (counteracts greenhouse gas warming). • Large uncertainty. My focus 12 Why is the indirect effect poorly understood? Indirect forcing uncertainties arise because: • Aerosol-cloud interactions take place at smaller spatial scales than climate models can resolve, and must be parameterized. • Aerosol-cloud interactions are complex; many aspects are unknown or poorly understood. • Climate models provide limited information about clouds, and aerosols. Central problem of indirect effect: Determine the relationship between aerosol and cloud radiative properties, using the limited information available by climate models. This problem has historically been reduced to finding the relationship between aerosol mass concentration and cloud droplet number concentration. 13 Droplet Concentration Current understanding: empirical (Boucher & Lohmann, 1995) Aerosol mass concentration • Very large variability. • Unresolved meteorology, cloud microphysics, aerosol chemical factors are responsible for the variability. • Need for physically-based parameterizations. 14 Desired approach: from first principles. Cloud droplet number balance in each grid box of the model: dN drop dt Qactivation Qevap Qadvection ... Activation is the direct aerosol-cloud droplet link. Embedding a numerical activation model is too slow; must use parameterization of activation. Parameterizations of aerosol activation have appeared in the literature over the years (from 1959). They are derived assuming: idealized cloud dynamics, aerosol composition and size distribution. Are current parameterizations good enough? (Hint: No). 15 Parameterizations: prescribed size distribution bias Fitting ambient size distributions to prescribed functional form introduces biases which can be important for indirect effect. This aerosol is “shifted” to larger sizes. 16 Current parameterizations: other weaknesses Lack of explicit treatment of mass transfer limitations in droplet growth; this has been shown to be important for polluted conditions (Nenes et al., 2001). Empirical correlations are used in many. They are derived from numerical simulations and can introduce biases when used outside their region of applicability. They lack important chemical effects that can influence cloud droplet formation. Such effects are the presence of : • slightly soluble species in the aerosol (Shulman et al., 1996) • water soluble gas-phase species (Kulmala et al., 1993) • surface tension changes from surface-active species in the aerosol (Facchini et al., 1999) • changes in water vapor accommodation coefficient from the presence of film-forming compounds (Feingold & Chuang, 2002) 17 Currently unaccounted “chemical” effects. Slightly soluble compounds: They add solute to the drop as it grows; this facilitates their ability to activate. Examples: organics (succinic acid), CaSO4. Soluble gases: They add solute to the drop as it grows; this facilitates their ability to activate. Examples: HNO3, HCl, NH3. A(g) A(aq) A(g) A(g) A(aq) A(aq) 18 Currently unaccounted “chemical” effects Surface-active soluble compounds: They decrease surface tension of droplets; this facilitates their ability to activate. Examples: organics (succinic acid, humic substances). Their solubility can be large. 75 Data of surface tension of concentrated samples from Tenerife clouds and Po Valley fogs -1 (kN m ) 70 65 60 (Charlson et al., 2001) 55 50 1e-4 1e-3 1e-2 1e-1 C(mol l-1) 19 Currently unaccounted “chemical” effects Film-forming compounds: They can slow down droplet growth. Once the film breaks, rapid growth is resumed: Film breaks water molecule water molecule Slow Rapid Examples: hydrophobic organics. Such substances do not alter droplet thermodynamics; kinetics of droplet growth are affected. If present, such substances can strongly affect droplet number. 20 Cloud droplet formation with film-forming compounds. log10(concentration) movie drop growth activation aerosol log10(size) 21 Chemical effects: assessment of their importance. Calculate the potential change in cloud properties when a chemical effect is present. 0.1 m s-1 0.3 m s-1 1.0 m s-1 3.0 m s-1 insoluble organic no D organic with D 5 ppb HNO3 x2 conc. marine aerosol 22 Chemical effects: summary, implications for parameterizations Chemical effects are seen to be important for many conditions. They can even be more effective than doubling aerosol concentrations (i.e. more effective than the “Twomey” effect). Chemical effects can be synergistic. One effect can be important for low updrafts (e.g. soluble gas effects) and another at higher updrafts (e.g. surface tension effects). This would lead to a systematic increase in droplet number for almost any cloud type. Lack of including them in activation parameterizations can lead to important uncertainties in indirect forcing. What does all this mean for current aerosol activation parameterizations? They are not adequate. We need to develop a new approach. 23 New parameterization: Underlying ideas section i d(Naerosol)/d(Size) Use sectional representation of aerosol chemistry and size distribution. Each section can: • have its own chemical composition • i-th section characterized by (i-1, i) boundaries • piecewise linear profiles between boundaries Size Multiple populations with their own distributions can co-exist and compete for water vapor. Modified Köhler theory for computing CCN properties. 24 New parameterization: conceptual framework Properties calculated from energy, mass balances. Adiabatic parcel model used to calculate droplet number. • Lagrangian framework of reference • Parcel properties are uniform t drop growth Smax activation • Constant updraft velocity • Parcel pressure is equal to ambient • Explicit treatment of mass transfer limitations of water vapor to droplet phase. aerosol S 25 New parameterization: Formulation Input: P,T, updraft velocity (cooling rate), RH, aerosol characteristics. Output: Droplet number How: Solve the algebraic equation (numerically) : wGSmax 2 aV Spart C1 0 f1 ( s)ds C2 f 2 ( s)ds 1 0 S p a rt S max i 2 part Ni Ai Sci i ln i 1 i 1 3 i 1 Sc Sc Sc G aV x Si 1/ 2 i part Ni S max x x 2 2 1/ 2 S x Arc sin max i i 1 2 S x Si1 2 i 1 S c S c RT M w L2 P sat M c p PM aT w RT L M wL G sat 1 P Dv M w kaT RT 1 26 Performance of new parameterization (200 test cases) 1.000 Activation ratio (New Parametrization) SM1 SM2 SM3 SM4 0.100 SM5 SM6 TM1 TM2 0.010 0.001 0.001 0.010 0.100 1.000 Activation ratio (Parcel Model) 27 Performance of existing parameterization (Ghan et al, 2000) 1.000 Activation ratio (Ghan Parametrization) SM1 SM2 SM3 SM4 0.100 SM5 TM1 TM2 0.010 0.001 0.001 0.010 0.100 1.000 Activation ratio (Parcel Model) 28 New parameterization: Marine aerosol with surfactants 0.70 Numerical Simulation Parameterization (with s.t.) Parameterization (without s.t.) Activation Fraction 0.60 0.50 0.40 0.30 0.20 0.10 0.00 0.1 1 10 Updraft Velocity (m/s) 29 New parameterization: assessment. A powerful activation parameterization for climate models has been developed for aerosol of: • “arbitrary” (non-ideal) size distribution, • complex chemical and size-dependant composition (surfactants, slightly soluble substances present). Furthermore, it • is fast (> 1000 times quicker than full numerical parcel model). • uses minimal amount of empirical information. • is more robust and accurate than other parameterizations in use. Some future directions: • incorporate other activation effects. • use it in a global model (GISS GCM with TOMAS). 30 New effect: Black Carbon heating Black carbon exists in polluted aerosol; it absorbs visible sunlight and heats the surrounding air. This can leads to decreased cloud coverage, and climatic warming. If black carbon is included in cloud droplets, the heat released can increase the droplet temperature enough to affect the droplet equilibrium. This is a new effect. drop BC core Absence of heating Presence of heating: droplet and gas phase get heated 31 New effect: Black Carbon heating BC heating can have an important effect on CCN of large dry diameter only. This results in three effects: 1. Inhibition of the activation of large CCN. This would make water vapor available for the activation of smaller (and more numerous) CCN. This tends to increase droplet concentrations and cool climate. 2. Release of heat into the gas phase drops parcel supersaturation. This tends to decrease droplet concentrations, and warm climate. 3. Decreased size of low Sc CCN can decrease the chance of drizzle formation (Giant CCN can initiate drizzle formation). This would tend to increase cloud lifetime and cool climate. Which of the mechanisms can prevail? 32 Black Carbon heating: effect on cloud albedo Consider a case where Mechanism 1,2 are strongest. 0.005 Simulation is for urban aerosol, 20% BC, 0.1 m s-1 updraft. Albedo difference 0.000 Mechanism 1 alone. -0.005 Conditions were chosen so that the heating released from the BC in each curve is the same. -0.010 Mechanism 1+2. -0.015 Albedo calculated by a twostream approximation. Differences are compared to absence of BC heating. Mechanism 2 alone. -0.020 -0.025 0 50 100 150 Cloud depth (m) Droplet changes are alone not significant but they can affect the magnitude of albedo change from BC heating. 33 Black Carbon heating: effect on drizzle formation. Assess the size of Giant CCN with and without BC heating, for a marine stratocumulus cloud. We use a Large Eddy Simulation of nonprecipitating marine stratocumulus (Stevens et al., J.Atmos.Sci, 1996). 500 Lagrangian trajectories derived from the LES are used to “drive” a cloud parcel model and grow the giant CCN in the cloud. Ensemble 1-hr averages of the parcel properties yield average cloud properties. 34 Black Carbon heating: 50 selected LES trajectories. Height Cloud movie Horizontal extent 35 Activation along one of the 500 trajectories. log10(concentration) movie log10(size) 36 Black Carbon heating: potential effect on drizzle 900 BC can effectively decrease the probability for drizzle formation. 500 parcel average 800 700 Cloud top A heating mechanism can lead to climatic cooling! Height (m) 600 500 Cloud base This effect can be parameterized (not shown). 400 300 200 Is it important? We don’t know yet. 0% BC, Pristine 10% BC, Pristine 20% BC, Pristine 100 0 0 10 20 30 GCCN average size (m) 40 50 37 CCN INSTRUMENTS: What do they do? Purpose: Measure the number of cloud droplets that can form for a variety of water vapor supersaturations (“CCN spectrum”), at a given point in space and time. Operation principle: Expose particle sample to a known water vapor supersaturation, and measure those that become droplets. Desired range: 0.01% - 1.0% supersaturation 38 CCN INSTRUMENTS: The need for theoretical analysis Objective: Assess the performance and limitations of current CCN measurement methodologies. Few designs available; we focus on spectrometers: • Most attractive for aircraft missions. • Minimal theoretical assessment of their capabilities. Aerosol sample Instantaneously CCN spectrum Approach: • Develop comprehensive models that express optimum instrument behavior. • Simulate instrument response, assuming that inlet aerosol is monodisperse. 39 Fukuta & Saxena (1979) CCN Spectrometer (FCNS) Outlet, droplet detection Metal envelope Insulation Inlet Metal envelope Hot tip Thot Insulation Tcold Smax Flow Wetted walls Cold tip Property (cross section) Thot-Tcold Smax Distance from tips 40 FCNS: How is it used? Inlet Characteristics: Each streamline has different S S constant along a streamline Droplet concentration Outlet Detection must distinguish droplets from unactivated aerosol. Usage: Measure droplet concentration at a (centerline) streamline. CCN = droplet concentration. Scan for all streamlines. Streamline supersaturation 41 FCNS: Mathematical model } Growth “driving force” Droplet phase: Gas phase: dD p dt S S eq (Lagrangian) Dp div u div S t transient advection diffusion (Eulerian) source Conservation Law S Continuity 1 0 0 x1 -momentum u x 2 -momentum v T ka cp C D v Heat Water vapor x 2a P x 2a x1 x1 u x1 P x 2a x 2a x2 x1 x2 u x2 D H vap cp a v x 2 x1 a v x 2 x 2 x2 J buoy av x2 J cond J cond 42 FCNS: Activation along different streamlines log10(concentration) movie Each color represents a different streamline CCN activate as they flow through the instrument log10(size) 43 FCNS: Activation at outlet of instrument poor Note: log10(concentration) fair Droplets separate well from aerosol at high S. good good separation aerosol Separation degrades as S decreases. droplets log10(size) 44 FCNS: Why does performance degrade as S decreases? Centerline supersaturation (%) 1.5 Outlet 1.0 High s: 1.05% (good) Inlet 0.5 0.0 -0.5 -1.0 -1.5 0.0 0.1 0.2 0.3 0.4 0.5 0.6 y-position Distance from entrance(m) of instrument (m) 0.7 45 FCNS: Why does performance degrade as S decreases? Centerline supersaturation (%) 1.5 Outlet 1.0 Inlet Medium s: 0.60% (good) 0.5 0.0 -0.5 -1.0 -1.5 0.0 0.1 0.2 0.3 0.4 0.5 0.6 y-position Distance from entrance(m) of instrument (m) 0.7 46 FCNS: Why does performance degrade as S decreases? Centerline supersaturation (%) 1.5 Outlet 1.0 Inlet 0.5 Low s: 0.14% (fair) 0.0 -0.5 -1.0 -1.5 0.0 0.1 0.2 0.3 0.4 0.5 0.6 y-position Distance from entrance(m) of instrument (m) 0.7 47 FCNS: Why does performance degrade as S decreases? Low S takes longer to develop than high S, and CCN are exposed to S > Sc for less time. Growth at low S is much slower than at high S; activation takes longer than at high S. Separation between droplets and non-activated aerosol is lost at low S. 48 CCN INSTRUMENTS: Assessment Fukuta CCN spectrometer: • sensitivity problems under low supersaturations. • optimum Sc range ~ 0.2% to 1.1% • not sensitive to aerosol chemical characteristics. All instruments displayed sensitivity problems for Sc < 0.08-0.1%. Some display large sensitivity to chemical composition. Model captures instrument behavior very well. Can be reliably used. Conclusion: • Instrumentation needs to be further improved. This is currently being done (Caltech, UCSD). • A deep theoretical understanding of any instrument is necessary for reliable CCN measurements. 49 GENERAL SUMMARY The indirect effect of atmospheric aerosols is one of the most important and challenging aspects of climate prediction science. A variety of aerosol activation effects need to be included in parameterizations of aerosol-cloud interactions. There are possibly many (counterintuitive) mechanisms to be discovered. Parameterizations are being developed, and included within a comprehensive climate model system. CCN instrumentation is a key tool for untangling the aerosol-cloud puzzle. Current instrumentation is not adequate in fulfilling its task. Source of problems identified. New CCN instruments under construction will. 50 ACKNOWLEDGMENTS People John Seinfeld, Caltech Rick Flagan, Caltech Bill Conant, Caltech Tracey Rissman, Caltech Graham Feingold, NOAA Bob Charlson, University of Washington Christina Facchini, Instituto ISAO, C.N.R. Steven Ghan, PNNL Peter Adams, Carnegie Mellon Greg Roberts, UCSD Funding EPA NASA ONR 51