* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Heart failure. Myocardial Infarction Ph.D., MD, Assistant Professor
Saturated fat and cardiovascular disease wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Rheumatic fever wikipedia , lookup
Electrocardiography wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Jatene procedure wikipedia , lookup
Heart failure wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Coronary artery disease wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
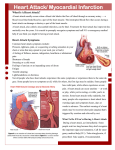
Heart failure. Myocardial Infarction Ph.D., MD, Assistant Professor Hanna Saturska Functions of circulatory system Stabilization of arterial pressure Tissue provision by О2 The vital role of the circulatory system in maintaining homeostasis depends on the continuous and controlled movement of blood through the thousands of miles of capillaries that permeate every tissue and reach every cell in the body. It is in the microscopic capillaries that blood performs its ultimate transport function. Nutrients and other essential materials pass from capillary blood into fluids surrounding the cells as waste products are removed. Heart insufficiency (Heart failure) Heart failure is the pathophysiologic state in which the heart, via an abnormality of cardiac function (detectable or not), fails to pump blood at a rate commensurate with the requirements of the metabolizing tissues or is able to do so only with an elevated diastolic filling pressure. Heart failure may be caused by myocardial failure but may also occur in the presence of nearnormal cardiac function under conditions of high demand. myocardial failure conditions of high demand Reasons Myocardium - Myocardium hypoxia or ischemia Infectional-toxical myocardium damage Metabolism disorder Nervous-trophical and hormonal influences on the organism Myocardium - - injury overload Increase of heart outflow resistance (heart aperture stenosis, arterial hypertension) Increase of diastolic inflow (hypervolemia, heart aperture insufficiency) Mixed Heart failure always causes circulatory failure, but the converse is not necessarily the case, because various noncardiac conditions (eg, hypovolemic shock, septic shock) can produce circulatory failure in the presence of normal, modestly impaired, or even supranormal cardiac function. To maintain the pumping function of the heart, compensatory mechanisms increase blood volume, cardiac filling pressure, heart rate, and cardiac muscle mass. However, despite these mechanisms, there is progressive decline in the ability of the heart to contract and relax, resulting in worsening heart failure. This chest radiograph shows an enlarged cardiac silhouette and edema at the lung bases, signs of acute heart failure. A 28-year-old woman presented with acute heart failure secondary to chronic hypertension. The enlarged cardiac silhouette on this anteroposterior (AP) radiograph is caused by acute heart failure due to the effects of chronic high blood pressure on the left ventricle. The heart then becomes enlarged, and fluid accumulates in the lungs (ie, pulmonary congestion). Heart failure can be classified into 4 classes Class I patients have no limitation of physical activity Class II patients have slight limitation of physical activity Class III patients have marked limitation of physical activity Class IV patients have symptoms even at rest and are unable to carry on any physical activity without discomfort Heart failure can be divided into 4 stages, as follows: Stage A patients are at high risk for heart failure but have no structural heart disease or symptoms of heart failure Stage B patients have structural heart disease but have no symptoms of heart failure Stage C patients have structural heart disease and have symptoms of heart failure Stage D patients have refractory heart failure requiring specialized interventions STAGES Compensation 1. Crash phase (main sense - compensative hyperfunction) 2. Stable adaptation phase (main sense - compensative hypertrophy) Decompensation 3. Exhaustion Crash phase (St. of compensation) Cardial mechanisms 1. HB increase (in 2,5 time) 2. Systolic volume increase 3. Heart index increase 4. Heart work increase Extracardial mechanisms 1. Increase of O2 utiliza-tion by the tissues 2. Reduce of peripheral vessels resistance Crash phase (St. of compensation) Reason increase of every cardiomyocytes load Physiological mechanisms * adequate excitement *relation of excitement and shortening * adequate shortening *energy provision Crash phase Immediate adaptation mechanisms 1. Adequate excitement Is based on selective penetration of Na+, K+, Са2+ due to difference between the extracellular ions concentration and intracellular one Result - depolarization Crash phase Immediate adaptation mechanisms 2. Relation of excitement and shortening *diffusion of depolarization wave inside the cardiomyocytes * Са2+ penetration in to cytoplasma from SPR * Са2+connection with troponin and release of myosin 3. Shortening *actin and myosin interaction Crash phase Immediate adaptation mechanisms 4. Energy provision *Glycolisis activation *Mitochondria activation *CrPh reserve, glycogen reserve(are localized on SPR membrane) -most sensitive - depolarization ( Na,K-АТPаse and Са- АТPаse control of ions transposition athwart concentration gradient Excessive Са concentration causes its accumulation in mitochondrias and block of АТP synthezise!!! Crash phase (pathogenesis) Heart beat increase Functional changes Increased penetration of Na and Са cytoplasma inside Decrease of depolarization interval Is possible if: activity of Na,K-ATPase and Са -ATPase is high CrPh reserve and ATP reserve is adequate ATP synthezise in mitochondrias is adequate Na,Са-regulative mechanism is adequate Crash phase (pathogenesis) Increase of shortening power ( heterometric mechanism and homeometric mechanism) Activation of adenilatcyclase by catecholamines cАМP synthesis Increase of Са concentration in cytoplasma Increase of free myosin fibers amount (Са blockades troponin) Increased amount of myosin-actin interaction Using of ATP, CrPh, glycogen Crash phase (pathogenesis) Limitation mechanisms 1. Accumulation of Na (because is limited Na,К-АТPase activity) 2. Violation of Na,Са-exchanged mechanism 3. Са accumulation (because limitation of Ca-АТPase activity) after-effect: cardiomyocyte relaxation deficit (diasole deficit) Са accumulation in mytochondrias (dissociation of oxidation and phocphorilation) 4. Energy deficit (deficit of АТP 40-60 % causes shortening depression) 5. Lactic acid accumulation (causes shortening depress ion because Н+ions interact with troponin) Crash phase (pathogenesis) Resume Limitation mechanisms cause condition when heart load is more than heart work. It is the sense of heart insufficiency. So, compensative hyperfunction as an adaptation mechanism is depleted Stable adaptation phase (stage of compensation) Gist: compensative hypertrophy Mechanisms * RNA synthesis activation in cardiomyocites * Increase of ribosome quantity in cardiomyocites * Structural proteins synthesis (at first mitochondrial proteins and SPR ones) * activation DNA and RNA synthesis in connective tissue cells of the heart (fibroblasts and endotheliocytes) * Controlled proliferation of the connective tissue cells (they are the donors of RNA and structural proteins) Result: heart stable adaptation to load Signs of hypertrophy Sick person 1. Continuous heart load 2. Heart hypertrophy is inadequate to body weight 3. Decrease of capillaries amount in weight unit 4. Inadequate activity of MCh 5. Inadequate activity of SPR 6. Decrease of nervous structures amount in weight unit (decrease of NA concentration) Sportsman 1. There are periods of heart load and restoring 2. Heart hypertrophy is adequate to body weight 3. Increase of capillaries amount in weight unit 4. Adequate activity of MCh 5. Adequate activity of SPR 6. Increase of nervous structures amount in weight unit (adequate concentration of NA) Signs of hypertrophy Sick person Results Heart insufficiency is compensated by the hypertrophy (bigger heart mass). But this change limits maximal heart work. Sportsman Results Heart insufficiency, which is compensated by the hypertrophy, increases of heart muscles contraction power and speed one. Heart work is increased and human endurances is increased too Exhaustion (stage of decompensation) Decrease of correlation between square cardiomyocyte surface and cardiomyocyte volume (unbalance of ions pumps) Decreased Na,K-АТPase activity (violation of repolarisation , appearance of arrhythmias) Decreased activity of SPR and СаАТPase (heart relaxes slowly, some time arise diastole defect at Са accumulation) Exhaustion (stage of decompensation) Decreased MCh activity and energy deficit because Са is accumulated in MCh and it causes dissociation of oxidation and phosphorilaion Depression of contractil function Exhaustion of connective tissue cells donors function Decrease of coronary blood flow reserve Decrease of NА concentration decrease of maximal speed shortening of the heart and maximal force one Exhaustion right-sided (stage of decompensation) left-sided Pathological signs Violations of blood circulation Reduce of systole output (increase of diastole excess blood volume, myogene dilation) Decrease of heart output Decrease of systole arterial pressure Increase of diastole arterial pressure Increase of veins pressure (causes the HR increase) Slowdown of blood flow (main sign of decompensation) Erythrocytosis (compensation) Pathological signs Breathing violations Dyspnoea (reflective irritation of breathing center by the СО2) Attacks of cardiac asthma at night (blood overflow of the atriums and central veins, which causes barro-receptors irritation and breathing center reflexes) Pathological signs Violation of water-electrolyte balance (edema) Blood circulation violation (slowdown blood flow in capillaries, intravenous blood pressure increase) Reflexes of blood circulation dumping (blood retention in depot : liver, veins) Deficit of blood circulation in the arteries Irritation of the vessels volume receptors Hypersecretion of aldosteron (Na retention) and vasopressin (water retention) Hypervolemia, ascytes, edema Myocardial infarction Ischemic heart disease occurs when there is a partial blockage of blood flow to the heart. When the heart does not get enough blood it has to work harder and it becomes starved for oxygen. If the blood flow is completely blocked then a myocardial infarction (heart attack) occurs. Myocardial infarction Ischaemical necrosis of the myocardial tissue, which is resulted from coronary blood supply insufficiency Statistics Morbidity increases Patients which suffer from myocardial infarction are younger year by year Mortality of the patients which suffer from myocardial infarction increases year by year (30-40 %) Coronary artery disease is currently the leading cause of death in the United States. Despite the increasing sophistication of surgical techniques, the introduction of new techniques such as balloon angioplasty, and a number of new drugs (e.g. beta blockers, calcium antagonists), it is estimated that over 1 million heart attacks will occur this year, resulting in 500,000 deaths. In short, we do not have an adequate therapeutic solution to the problem of myocardial infarction (heart attack). ЕТHІОLOGY Atherosclerosis of the coronary arteries (in 90-95 % died persons at section was found) Trombosis of the coronary arteries : *at 4 stage of atherosclerosis *arterial hypertension (because it causes blood coagulation hyperactivity) Trombembolism (septic endocarditis, thrombus lyses) Spasm of the coronary arteries Risk factors 1. Stress (at trauma, operation, cold, negative emotions) BECAUSE IT CAUSES: Increase of the heart activity Stimulation of the heart metabolism Increase of О2 using Risk factors 2. Age (most often appears in 40 – 59 years old person). 3. Hypokinesia (activation of the sympathetic-adrenal system) 4. Obesity (hypercholesterolemia) Risk factors 5. MAIL SEX Morbidity of the men in 2-3 time more Mortality of the men in 3-4 time more Men 45-59 years old - mortality 37 % Woman 45-59 years old – mortality 17 % Men 60-74 years old - mortality 55 % Woman 60-75 years old – mortality 78,4 % Risk factors 6. Heredity 7. Arterial hypertension 8. Diabetes mellitus 9. Infection (chlamydia pneumonia) Pathogenesis 1. 2. Initial mechanisms Mechanisms of the cardiomyocites necrosis As a result of atherosclerotic disease of the coronary arteries As a result of cardiomyocytes ischemia Initial mechanisms 1. Increase of the atherosclerotical plaque size: Vessel narrowing---ischemia---necrosogenic ATP deficit vessels narrowing on 95 % (“critical stenosis”) causes АТP deficit (less than 40-60 %) which results in cardiomyocytes necrosis Initial mechanisms 2. Increase of injured vessel sensitivity to vasospastic effects Damage of endothelium ----decrease of NО-synthetase activity---decrease of NО concentration (which is powerful vasodilator) Initial mechanisms 3. Thrombosis Anticoagulants blood activity decrease (heparin is used for activation of lipoprotein lipase at hyperlipoproteinemia) Decreased antithrombosis properties of the injured endothelium Unmasked collagen fibers cause activation of the Villebrand’s factor Cardiomyocytes necrosis mechanism 1. ATP deficit Decrease of the cytochromoxydase activity Violation of electrons transfer in MCh Violation of Krebs-cycle Accumulation of acetylcoensime-A, fat acids Deficit of ATP and CPh causes - ineffective Na,К-АТPase (fatal arrhythmias) - ineffective Са-АТPase (damage of the Cardiomyocytes necrosis mechanism 2. Acidosis Accumulation Accumulation Accumulation Accumulation Accumulation of of of of of Crebs-cycle metabolits Acetyl-Co-A fatty acids piruvate acid lactic acid Cardiomyocytes necrosis mechanism Acidosis after-effects **depression of cardiomyocytes contractility (main sign of ischemical area) Mechanisms 1. Н+-ions interact with troponin. It causes of myosin releasing impossibility. So, as a result, interaction of actin and myosin becomes impossible 2. Са deficit in cytoplasma occurs because Ca can be accumulated in Mch very often it is complicated by the “reperfusion syndrome” Cardiomyocytes necrosis mechanism 3. Са accumulation Reasons: 1. Deficient of Ca return in to SPR (ATP deficit decreases Ca-ATPase activity) 2. Violation of Na,Са-exchange mechanism Consequences: Ca deposit in Mch and АТP deficit Damage of cardiomyocytes membranes Cardiomyocytes necrosis mechanism 4. “Lipid triade” 1. Phospholipase activation (is caused by catecholamines and Ca) 2. Lipids peroxidation (accumulation of the free radicals, relative insufficiency of the antioxidants) 3. Fat acids (damage of the membrane’s lipids and violation of the ion channel’s functions) Hibernal myocardium Especial condition of the heart which is characterized by the sharply decreased pump function of the heart (at human absolute rest) without cardiomyocytes cytolysis as a result of blood supply reducing (protective reaction) Hibernal myocardium Sings Decreased left ventricle output at increased O2 need of the organism (physical activity, fever, hyperthyroidism) Decreased using of ATP Retardation of the cardiomyocytes necrosis Renewal of Н+ concentration, creatinphosphate level, рСО2 (during 1-3 hour) Hibernal myocardium Finishing Spontaneous recurrent process after blood supply restoring !!! 1 stage – hypokinetic and asynchronous cardiomyocytes contruction 2 stage – renewal of synchronous cardiomyo-cytes contruction and left ventricle output rising at increased O2 need of the organism (physical activity) The chronic but reversible myocardial dysfunction seen in patients with severe coronary artery disease is a complex, progressive, and dynamic phenomenon that is initiated by repeated episodes of ischemia. In the early stages, resting perfusion is usually preserved, but flow reserve is significantly reduced. With time, and probably also increases in the physiological significance of the underlying coronary narrowing, some of the dysfunctional segments which initially appeared “chronically stunned” may eventually become underperfused, probably in response to the decrease in myocyte energy demand. This transition from chronic stunning to chronic hibernation is associated with several morphological alterations, which include myofibrillar disassembly, myofibrillar loss, and increased glycogen content. Interestingly, these changes take place similarly in dysfunctional and in normally perfused remote regions of the dysfunctional heart, which suggests that they may be more a response to chronic elevations in preload or stretch than the direct consequences of ischemia. (Panel A): Light micrograph of a normal myocardium. (Panel B): Representative light micrograph of hibernating myocardium. The myolytic cytoplasm is filled with PAS-positive material typical of glycogen. Magnification ´320. Myocardial Infarction Prevention Strophanthin comes from an extract of an African plant called strophanthus gratus. Since 1991 it was discovered as an endogenous substance that research shows can prevent angina pectoris and myocardial infarction by 80-100 percent without major side effects. strophanthus gratus