* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Heart Rhythm Refresher Course 2014 Module 1: Epidemiology
Electrocardiography wikipedia , lookup
Saturated fat and cardiovascular disease wikipedia , lookup
Turner syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Antihypertensive drug wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Marfan syndrome wikipedia , lookup
Cardiovascular disease wikipedia , lookup
Cardiac arrest wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Coronary artery disease wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
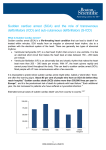
Dr Yue Chiu Sun Consultant and Head Division of Cardiology Dept of Medicine & Geriatrics United Christian Hospital 6 Apr 2014 • Sudden cardiac death (SCD) is an unexpected death due to cardiac causes that occurs in a short time period (generally within 1 hour of symptom onset) in a person with known or unknown cardiac disease • SCD represents the first expression of cardiac disease in many individuals who experience out-of-hospital cardiac arrest • ~80-90%: VT and VF • survival rate for out of hospital arrest <5%. Epidemiology of SCD It is estimated that more than 7 million lives per year are lost to SCD worldwide, including ~325,000 in the United States (incidence of ~0.1-0.2%/year in adult population) In Hong Kong, more than one thousand people lose their lives suddenly and unexpectedly every year. Many cases are due to ventricular fibrillation VF causing sudden cardiac arrest Sudden Death U.S.A. 100-200 / 100,000 / year Japan 68-104 / 100,000 / year Netherlands 100 / 100,000 / year Greece 90 / 100,000 / year Taiwan 58~73 / 100,000 / year Iceland 56 / 100,000 / year Finland 48 / 100,000 / year Israel 46 / 100,000 / year England 40 / 100,000 / year Huang et al. Acta Cardiol Sin 2006 Epidemiology of sudden cardiac death in Asia Sudden cardiac death (SCD) occurs in ~40 cases per 100,000 persons annually in each country of Asia Most cases are caused by MI and VF in out-of-hospital cardiac arrest cases, but the proportion of myocardial infarction is lower in Asia than in Western countries The primary electrophysiological disorders related to channelopathies, such as long QT syndrome, short QT syndrome, Brugada syndrome, early repolarization syndrome, and catecholaminergic polymorphic ventricular tachycardia (CPVT) are estimated to be responsible for 10% of SCDs Circ J. 2013;77(10):2419-31 Incidence and Demographics of Sudden Death in Hong Kong The first territory-wide survey on sudden death in the Hong Kong SAR was conducted by the Cardiology Division, Department of Medicine, HKU an incidence of 18 sudden deaths per 100,000 population in the year 1997 Out of 1204 cases of out-of-hospital sudden deaths reported to the coroner, the underlying causes were -AMI in 31%, CAD in 26%, HT heart diseases in 14%, ruptured aortic aneurysm in 6%, CMP in 5%, other CV diseases in 7%, non-CV causes in 11% Causes of SCD Causes of SCD depend on age, gender, ethnicity, and genetics Age < 40 yrs: common causes include hypertrophic cardiomyopathy (HCM), unexplained left ventricular hypertrophy, myocarditis, congenital heart disease and less commonly aortic dissection, valvular heart disease and arrhythmogenic right ventricular dysplasia/ cardiomyopathy (ARVD/C) Age > 40 yrs: atherosclerotic coronary heart disease is the most common cause Causes of SCD Under one year of age, SCD can be part of sudden infant death syndrome (SIDS) which is often associated with a negative postmortem Sudden death with a negative postmortem is referred to as “autopsy-negative sudden unexplained death (SUD)”, the cause of which is most likely a primary arrhythmogenic event Genetics of SCD The inherited structural cardiovascular diseases causing SCD are most frequently HCM, ARVC and dilated cardiomyopathy On the other hand, the most likely encountered inherited arrhythmogenic disorders that may cause SCD are congenital long QT syndrome (LQTS), Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia (CPVT) and short QT syndrome (SQTS) Genetics of SCD Postmortem molecular diagnostic studies, referred to as “molecular autopsy”, have identified pathogenic mutations in LQTS, Brugada syndrome, CPVT and SQTS-related genes in over one third of SCD victims -> inherited arrhythmogenic disorders : ion channelopathies Genetics of SCD- Brugada syndrome Brugada syndrome has been linked to mutations in the SCN5A gene that encode the –subunit of the sodium channel and those in the CACNA and CNCNB genes that encode the -and -subunits of the calcium channel respectively and the gene that encodes glycerol-3-phosphate dehydrogenase 1-like enzyme GPD-L However, more than 70% of cases remain unaccounted for by the genotype In Japan, the incidence of SCN5A mutations varies from 2% to 27% (mean 12%) which is much lower than that reported in Europe (20%) Brugada phenotype is 8 to 10 times more prevalent in men than in women This gender distinction is attributed to the presence of a more prominent Ito giving rise to a more prominent notch in the action potential of right ventricular epicardial cells in males versus females Ethnicity of SCD Most studies demonstrate inconclusive data with regard to racial differences as they relate to the incidence of sudden death. Some studies suggest that a greater proportion of coronary deaths were "sudden" in blacks compared to whites Gender difference Men have a higher incidence of SCD than women, with a ratio of 3:1 This ratio generally reflects the higher incidence of obstructive coronary artery disease in men Recent evidence suggests that a major sex difference may exist in the mechanism of myocardial infarction. - men tend to have coronary plaque rupture - women tend to have plaque erosion Sudden death in Atheletes The incidence of SCD among young athletes is estimated to be 1–3 per 100,000 person years, is higher than in non-athletes Br J Sports Med 2009;43:644–648 Causes of Sudden Death in young athletes Hypertrophic cardiomyopathy HCM Van Cam et al.(%) Maron et al.(%) 51 36 Possible death due to HCM 5 10 Coronary artery anomalies 16 19 Myocarditis 7 3 Aortic valve stenosis 6 4 Dilated cardiomyopathy 5 3 Aortic rupture 2 5 Arrhythmogenic right ventricular cardiomyopathy ARVC 1 3 Atherosclerotic coronary artery disease 3 2 Others 4 15 100 100 Total Athletes Recommendations Class I 1. Pre-participation history and physical examination, including family history of premature or sudden death and specific evidence of cardiovascular diseases such as cardiomyopathies and ion channel abnormalities is recommended in athletes. (Level of Evidence: C) 2. Athletes presenting with rhythm disorders, structural heart disease, or other signs or symptoms suspicious for cardiovascular disorders, should be evaluated as any other patient but with recognition of the potential uniqueness of their activity. (Level of Evidence: C) 3. Athletes presenting with syncope should be care-fully evaluated to uncover underlying cardiovascular disease or rhythm disorder. (Level of Evidence: B) 4. Athletes with serious symptoms should cease competition while cardiovascular abnormalities are being fully evaluated. (Level of Evidence: C) Class IIb 1. 12-lead ECG and possibly echocardiography may be considered as preparticipation screening for heart disorders in athletes. (Level of Evidence: B) ACC/AHA/ESC 2006 Guidelines Prevention of SCD at Community Level 1. Recognition of risk factors for CV disease 2. 3. 4. 5. appropriate treatment and control Recognition of high risk population for SCD Maintain healthy lifestyle Public education Pre-participation CV screening of athletes 1) Recognition of risk factors for CV disease treatment and control Smoking Dyslipidemia Hypertension Diabetes Obesity Family history of premature coronary artery disease Sedentary lifestyle Treatment and Control Risk Factor Modification Healthy diet Regular exercise Weight reduction (IBW) Smoking cessation Medical Therapy Beta blockers ACE inhibitors / ARB Lipid-lowering therapy Medications for DM, HT Interventional Procedures Implantable cardioverter defibrillator (ICD) Revascularization therapy Ablative therapy 2) Recognition of high risk population for SCD Survivors of sudden cardiac arrest Sustained VT Prior Myocardial Infarction with low LVEF, NSVT and inducible VT/VF during EPS Heart Failure (Class III to IV) LV Ejection Fraction < 35 - 40% Family History of Sudden Cardiac Arrest Others: Recurrent unexplained syncope and significant LV dysfunction , HCM or ARVC who have risk factor(s) for SCD, longQT syndrome, Brugada syndrome with syncope or VT, CPVT 2012 ACCF / AHA / HRS Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities ICD Therapy Class I 1 ICD therapy is indicated in patients who are survivors of cardiac arrest due to VF or hemodynamically unstable sustained VT after evaluation to define the cause of the event and to exclude any completely reversible causes. (Level of Evidence: A) 2 ICD therapy is indicated in patients with structural heart disease and spontaneous sustained VT, whether hemodynamically stable or unstable. (Level of Evidence: B) ICD therapy Class I 3 ICD therapy is indicated in patients with syncope of undetermined origin with clinically relevant, hemodynamically significant sustained VT or VF induced at electrophysiological study. (Level of Evidence: B) 4 ICD therapy is indicated in patients with LVEF less than 35% due to prior MI who are at least 40 days post-MI and are in NYHA functional Class II or III. (Level of Evidence: A) (1º prevention) 5 ICD therapy is indicated in patients with non-ischemic DCM who have an LVEF less than or equal to 35% and who are in NYHA functional Class II or III. (Level of Evidence: B) (1º prevention) ICD therapy Class I 6 ICD therapy is indicated in patients with LV dysfunction due to prior MI who are at least 40 days postMI, have an LVEF less than 30%, and are in NYHA functional Class I. (Level of Evidence: A) (1º prevention) 7 ICD therapy is indicated in patients with non-sustained VT due to prior MI, LVEF less than 40%, and inducible VF or sustained VT at electrophysiological study. (Level of Evidence: B) ICD therapy – Class IIa 1 ICD implantation is reasonable for patients with unexplained syncope, significant LV dysfunction, and non-ischemic DCM. (Level of Evidence: C) 2 ICD implantation is reasonable for patients with sustained VT and normal or near-normal ventricular function. (Level of Evidence: C) 3 ICD implantation is reasonable for patients with HCM who have 1 or more major risk factors for SCD. (Level of Evidence: C) 4 ICD implantation is reasonable for the prevention of SCD in patients with ARVD/C who have 1 or more risk factors for SCD. (Level of Evidence: C) ICD therapy – Class IIa 5 ICD implantation is reasonable to reduce SCD in patients with long-QT syndrome who are experiencing syncope and/or VT while receiving beta blockers. (Level of Evidence: B) 6 ICD implantation is reasonable for non hospitalized patients awaiting transplantation. (Level of Evidence: C) (from Class IIb) 7 ICD implantation is reasonable for patients with Brugada syndrome who have had syncope. (Level of Evidence: C) (from Class IIb) ICD therapy – Class IIa 8 ICD implantation is reasonable for patients with Brugada syndrome who have documented VT that has not resulted in cardiac arrest. (Level of Evidence: C) 9 ICD implantation is reasonable for patients with catecholaminergic polymorphic VT who have syncope and/or documented sustained VT while receiving beta blockers. (Level of Evidence: C) 10 ICD implantation is reasonable for patients with cardiac sarcoidosis, giant cell myocarditis, or Chagas disease. (Level of Evidence: C) 3. Maintain healthy lifestyle 4. Public education i) Regular body check ii) Bystander CPR iii) AED (Automated External Defibrillator) iv) HK-IN-PACE: Sudden Cardiac Death Public Awareness Campaign Bystander CPR For adults with sudden out-of-hospital SCA, compression-only bystander CPR (without rescue breathing) appears to have equal or possibly greater efficacy compared with standard bystander CPR (compressions plus rescue breathing). The 2010 American Heart Association (AHA) Guidelines for CPR recommended that bystanders perform compression-only CPR to provide high-quality chest compressions prior to the arrival of emergency personnel 時間是復甦搶救成功的關鍵 100 發現心臟驟停 1 分鐘 急救車到達現場 1 分鐘 致電 999 1 分鐘 80 進行自行急救 6 分鐘 確認病者並進行電擊 成功率每分鐘下降 7-10% 90 2分鐘 總時間 11分鐘 % 復甦成功率 70 60 50 40 缺腦 氧細 ,胞 損 傷 30 20 10 0 0 1 2 3 4 5 6 7 8 9 時間 (分鐘) 美國心臟協會建議, 在醫院以外發生心臟驟停後3-5分鐘內要進行除顫 10 自動體外除顫器 AED (Automated External Defibrillator) AED 11 2 3 HKCC AED program 2008 Aim: increase public awareness, promote layperson training in basic life support skill, and coordinate AED installation in suitable locations Hong Kong College of Cardiology (HKCC) donated the first public-access AED to Lan Kwai Fong in 2007 Public-access AED has been proven in clinical trials that it saved 50% more lives from out of-hospital cardiac arrest 5. Pre-participation Cardiovascular Screening of Competitive Athletes Both the American Heart Association (AHA 2007) and the European Society of Cardiology (ESC 2005) have proposed guidelines for pre-participation screening for young athletes planning to begin competitive sports. The most significant difference between these two proposals is that the AHA guidelines include a preparticipation history and physical examination without further routine testing, while the ESC proposal includes a standard 12-lead ECG in all patients. Pre-participation Cardiovascular Screening of Competitive Athletes the incidence of SCD among competitive athletes is actually quite low, estimated to be between 1 per 50,000 athletes to 1 per 300,000 athletes over a 10 to 20 year period the incidence of SCD among marathon runners is low (one death per 215,000 hours) but is higher than that for other exercise, such as non-competitive jogging (one death per 396,000 hours), cross-country skiing (one death per 607,000 hours), or general, noncompetitive exercise (one death per 375,000 hours) AHA Recommendations for Pre-participation Cardiovascular Screening of Competitive Athletes 2007 Medical history: 1. Exertional chest pain/discomfort 2. Unexplained syncope/near-syncope† 3. Excessive exertional and unexplained dyspnea/fatigue, associated with exercise 4. Prior recognition of a heart murmur 5. Elevated systemic blood pressure Family history: 6. Premature death (sudden and unexpected, or otherwise) before age 50 years due to heart disease, in 1 relative 7. Disability from heart disease in a close relative 50 years of age 8. Specific knowledge of certain cardiac conditions in family members: hypertrophic or dilated cardiomyopathy, long-QT syndrome or other ion channelopathies, Marfan syndrome, or clinically important arrhythmias Physical examination: 9. Heart murmur‡ 10. Femoral pulses to exclude aortic coarctation 11. Physical stigmata of Marfan syndrome 12. Brachial artery blood pressure (sitting position) ESC 2005 Pre-participation Cardiovascular Screening of Competitive Athletes Controversy has evolved over the most practical and effective strategy for preparticipation cardiovascular screening of competitive athletes to detect unsuspected cardiovascular disease and prevent sudden death on the athletic field Athlete screening in the Veneto region of Italy is part of a national program (with 12lead ECG) that has reported the detection of previously undiagnosed hypertrophic cardiomyopathy and a decrease in the cardiovascular death rate in young athletes. In this study, over time periods of similar length, cardiovascular-related mortality rates in Veneto athletes were compared with those of a demographically similar region of the United States (Minnesota) in which screening is limited to history and physical examination There were 55 sudden CV deaths reported in Veneto over 26 years (2.1/year), compared with 22 deaths in 23 years (0.96/year) in Minnesota. Over the recent and comparable 11-year period, 1993 to 2004, 12 deaths were reported in Veneto and 11 in Minnesota. When analyzed as deaths per 100,000 person-years, Veneto exceeded Minnesota for all years combined (1.87 for 1979 to 2004 vs 1.06 for 1985 to 2007, respectively, p = 0.006), although the 2 regions did not differ significantly for 1993 to 2004 (0.87 vs 0.93, respectively, p = 0.88) or most recently for 2001 to 2004 (0.43 vs 0.90, respectively, p =0.38) Pre-participation Cardiovascular Screening of Competitive Athletes In conclusion, sudden CV deaths in young competitive athletes occurred at a low rate in both Veneto and Minnesota. Despite different pre-participation screening strategies, athlete sudden death rates in these demographically similar regions of the United States and Italy have not differed significantly in recent years These data do not support a lower mortality rate associated with pre-participation screening programs involving routine ECG and examinations by specially trained personnel Am J Cardiol. 2009 Jul 15;104(2):276-80 Recreational athletes In general, patients with known genetic disorders that predispose to SCD (eg, hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, Marfan syndrome, long QT syndrome) should avoid recreational activities with the following characteristics: -"Burst" exertion, involving rapid acceleration and deceleration, as is common in sprints, basketball, tennis, and soccer. Activities with stable energy expenditure, such as jogging, biking on level terrain, and lap swimming are preferred. - Extreme environmental conditions (temperature, humidity, and altitude) that impact blood volume and electrolytes. - Systematic and progressive training focused on achieving higher levels of conditioning and excellence. Patients with unusual or high-risk clinical features may require greater restriction. These features include a history of syncope or presyncope, prior cardiac surgery, prior arrhythmic episodes, or an implantable cardioverterdefibrillator (ICD).