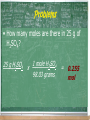* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download +2 - Fort Thomas Independent Schools
List of phenyltropanes wikipedia , lookup
History of chemistry wikipedia , lookup
Host–guest chemistry wikipedia , lookup
Rigid rotor wikipedia , lookup
Coordination complex wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Stoichiometry wikipedia , lookup
Organic chemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
Chemical bond wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Molecular dynamics wikipedia , lookup
Metalloprotein wikipedia , lookup
Drug discovery wikipedia , lookup
Debye–Hückel equation wikipedia , lookup
Homoaromaticity wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Gas chromatography–mass spectrometry wikipedia , lookup
Atomic theory wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Ionic compound wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Modern Chemistry
Chapter 7
Chemical Formulas and
Chemical Compounds
1
Section 1
Chemical Names
and Formulas
2
Chemical Formula
Eight carbon
atoms in the
molecule
Eighteen
hydrogen atoms
in the molecule
Molecular compounds – for one molecule
Ionic compounds – for one formula unit; the
simplest ratio of cations to anions
3
Chemical Formula
2 Al atoms
4 O atoms
4 SO4 ions
5 ions – two Al 3+ ions and three (SO4)
2-
ions
4
Definitions
• Binary Compound – compound with
only two types of atoms
• Nomenclature – a naming system
• Salt- an ionic compound composed of
a cation and an anion from an acid
5
Definitions
• Monatomic ions – ions formed from a
single atom
• For negative monatomic ions, -ide is
added to the root name
6
How can I tell if the compound
is ionic or covalent ????
Ionic compounds
contain
metal
and a nonmetal or
a
a
polyatomic ion !!!
7
Writing Fomulas and Naming
Ionic Compounds
8
Charges on Monatomic Ions
+
1
2+
+
3
32 1
9
Charges on Transition Metals
Ag
Zn
Cu
Fe/Cr
Sn
Pb
See list.
1+
2+
1+
2+
2+
2+
or
or
or
or
2+
3+
4+
4+
10
Charges of Metals
PbO2 and PbO
11
Polyatomic Ions Page 226
12
Formulas
Ionic Compounds
1. Write the symbols
2. Determine the charges
1. Monatomic ions from the periodic table
2. Transition metals from a roman numeral
3. Polyatomic ions from sheet.
3. Cross the charges.
4. Reduce to lowest ratio.
13
Names
Ionic Compounds
1. Write the cation name.
2. Write the anion name.
• Add –ide to the anion if monatomic
3. Add the roman numeral for any
transition metal.
4. NO PREFIXES!!!!
14
Ionic Binary Compounds
FORMULAS
Aluminum Oxide
3+
(3+ x
2
) + (2- x
2-
3 )=0
Al2O3
(charge x subscript)+(charge x subscript) = 0
15
FORMULAS
Ionic Binary Compounds
Aluminum Oxide
3+
2-
Al2O3
16
NAMING
Ionic BinaryCompounds
Mg3N2
ide
Magnesium Nitrogen
17
Practice Page 223
1. Write formulas for the binary ionic compounds
formed between the following elements:
a. potassium and iodine
b. magnesium and chlorine
c. sodium and sulfur
d. aluminum and sulfur
e. aluminum and nitrogen
2. Name the binary ionic compounds indicated by
the following formulas:
a. AgCl
e. BaO
b. ZnO
f. CaCl2
c. CaBr2
d. SrF2
18
FORMULAS
Ionic Compounds with Transition Metals
Iron (III) Chloride
3+
(3+ x
- x
)
+
(1
1
1-
3 )=0
FeCl3
19
FORMULAS
Ionic Compounds with Transition Metals
Tin (II) Oxide
2+
(2+ x
- x
)
+
(2
1
2-
1 )=0
SnO
20
FORMULAS
Ionic Compounds with Transition Metals
Tin (II) Oxide
2+
2-
SnO
21
Practice Page 225
1. Write the formula and give the name for
the compounds formed between the
following ions:
a. Cu 2+ and Br −
d. Hg 2+ and S 2−
b. Fe 2+ and O 2−
e. Sn 2+ and F −
c. Pb 2+ and Cl −
f. Fe 3+ and O 2−
2. Give the names for the following
compounds:
a. CuO
c. SnI4
b. CoF3
d. FeS
22
NAMING
Ionic Compounds with Transition Metals
PbO
( 2+ x 1 ) + ( 2 - x 1 ) = 0
Lead
II
Oxygen
ide
23
FORMULAS
Ionic Compounds with Polyatomic Ions
Ammonium Sulfate
+
(1+ x
2
) + (2- x
2-
1 )=0
(NH4)2SO4
Parenthesis are needed if the p. ion has a subscript
from the crossed charge – outside the ( ).
24
NAMING
Ionic Compounds with Polyatomic Ions
Ca(NO3)2
Calcium Nitrate
25
Practice Page 227
1. Write formulas for the following ionic
compounds:
a. sodium iodide
e. copper(II) sulfate
b. calcium chloride
f. sodium carbonate
c. potassium sulfide
g. calcium nitrite
d. lithium nitrate
h. potassium perchlorate
2. Give the names for the following compounds:
a. Ag2O
b. Ca(OH)2
c. KClO3
d.NH4OH
e. Fe2(CrO4)3
f. KClO
26
Writing Fomulas and Naming
Molecular Compounds
27
p. 228
28
Prefixes for Covalent
Molecular Compound
Formulas
1. Write the symbols.
2. Use prefixes for subscripts.
3. DON’T reduce.
29
Names
Molecular Compound
1. Write the name of the elements.
– Order: Smaller group number first;
– Same group? Greater period number
first.
2. Add –ide to the second element.
3. Add prefixes to each element for the
number of atoms.
30
FORMULAS
Binary Molecular Compounds
Disulfur difluoride
S2F2
31
NAMING
Binary Molecular Compounds
di nitrogen
tetra oxygen
ide
32
Practice Page 229
1. Name the following binary molecular
compounds:
a. SO3
b. ICl3
c. PBr5
2. Write formulas for the following
compounds:
a. carbon tetraiodide
b. phosphorus trichloride
c. dinitrogen trioxide
33
Acids and Salts
• Acids – Chapter 14
• Binary Acids – two elements; hydrogen
and one other element
• Oxyacids – contain hydrogen, one
other element and oxygen
• Acid – typically thought of as an H
donor; usually referred to as a solution
of the compound in water.
34
Acids and Salts
• Salt – an ionic compound
• Made from
– the cation of a base and
– the anion of an acid
• Some retain an H in the anion
– Example: CO3 2- carbonate
HCO3 1- hydrogen carbonate
or bicarbonate
35
p. 230
36
Salt Definition Animation
List of Acids
p. 230
Know these acids:
HF, HCl, H3PO4,
HNO3, H2SO4
CH3COOH
37
Section 3
Oxidation
Numbers
38
Oxidation Numbers
• Oxidation numbers are numbers assigned
to the atoms in a molecular compound or
ion that indicates the general distribution
of electrons among bonded atoms.
• Oxidation numbers are not actual charges.
• Oxidation numbers can be useful in naming
compounds and writing formulas.
+2
-1
+1
+3
-2
Rules for Assigning Oxidation
Numbers
– Atoms in a pure element have an
oxidation number of zero – O2 Ox. # = 0
– Fluorine always has ox. # of -1
– Oxygen almost always has ox. # of -2
except in peroxides such as H2O2 – then
it is a -1.
Rules for Assigning Oxidation
Numbers
• (Rules continued):
– Hydrogen’s ox. # is +1 unless it is with
metals – then it is -1
– The sum of the ox. # in molecules must
be zero, but in polyatomic ions, it is
equal to the ions charge.
Oxidation Numbers
• What are the oxidation numbers for each atom in
these compounds?
UF6 :
Fluorine is -1 x 6 = -6
Uranium +6
{+6 + (-6)} = 0
H2SO4 :
Oxygen is -2 (x 4 = -8)
Hydrogen is +1 (x 2 = +2) so
Sulfur has to be +6
{ (+6) + (+2) + (-8) }= 0
Oxidation Numbers
• What are the oxidation numbers for the
chlorate polyatomic ion?
ClO3- : Oxygen is -2 x 3 = -6
Chlorine must be +5
{ (+5) + (-6)} = -1 (the ion’s
charge)
Section 4
Using Chemical
Formulas
44
Formula Mass
• With a chemical formula, you can
calculate many characteristic values for a
compound.
• Formula Mass:
– Compounds have masses – just like
elements.
Formula Mass
• Formula Mass:
– The formula mass of any molecule,
formula unit, or ion is the sum of the
average atomic masses of all the atoms
represented in its formula.
– To find the mass of a compound simply
add the masses of the atoms that make
up the compound. Units are amu’s.
Formula Mass
• To find the formula mass of sulfuric acid
(H2SO4):
element # of atoms x mass (to 2 decimals)
H
2
1.01 = 2.02 amu
S
1
32.01=32.01 amu
O
4
16.00=64.00 amu
98.03 amu
Formula Mass
• To find the formula mass of Calcium
Nitrate Ca(NO3)2
element # of atoms x mass =
Ca
1
40.08 =40.08 amu
N
2
14.01 =28.02 amu
O
6
16.00= 96.00 amu
164.10 amu
Molar Mass
• Molar Mass
– The mass of a mole of any substance is
equal to its formula mass – except instead
of amu’s it is in grams.
– Formula mass of sulfuric acid = 98.03 amu
– Molar mass of sulfuric acid = 98.03 grams
Molar Mass
• To find the molar mass of Calcium Nitrate
Ca(NO3)2
element # of atoms x mass =
Ca
1
40.08 =40.08 g
N
2
14.01 =28.02 g
O
6
16.00= 96.00 g
164.10 g
Percentage Composition
• It is sometimes useful to know what the
percentage of a compound is an element.
• What percentage of water is oxygen?
H: 1.01 x 2 = 2.02
O: 16.0 x 1 = 16.0
Molar Mass= 18.02 g
16.0 ÷18.02
= 88.79%
Molar Mass
• Molar Mass can be used as a
conversion factor.
1 mole H2SO4
or
98.03 grams
98.03 grams
1 mole H2SO4
Problems
• How many moles are there in 25 g of
H2SO4?
25 g H2SO4
1 mole H2SO4
x
98.03 grams
= 0.255
mol
Problems
• What is the mass of 4.2 moles of
H2SO4?
4.2 mol H2SO4
x
98.03 g H2SO4
1 mol H2SO4
= 411.73
mol
Problems
• How many molecules are in 54 g of
H2SO4?
54 g H2SO4
x
6.02 x 1023 molecules H2SO4
98.03 g H2SO4
3.32 x 1023
molecules
=
Section 4
Determining Chemical
Formulas
56
Chemical Formula from % Comp
• Check periodic table for molar masses of each
element
• Find moles of each element present (assume
100g since we are working with percentages)
• To determine the simplest ratio of moles in the
compound, select the smallest number of moles
calculated and divide the other moles calculated
by that number
• Write the formula using the smallest whole
number ratio of elements
Empirical formulas
• Simplest formula
• Consists of element symbols and
subscripts showing the numbers of each
element
• Really, subscripts represent the smallest
whole number ratios
Empirical formulas
What is the empirical formula of a
compound containing 56.6% K, 8.7% C
and 34.7 % O ?
K= 39.10 g/mol
C = 12.01 g/mol
O = 16.00 g/mol
Empirical formulas
K 56.6 g K x 1 mol K
39.10 g K
C 8.7 g C x 1 mol C
12.01 g C
O 34.7 g O x 1 mol O
16.00 g O
Smallest value = 0.724
by this number.
= 1.45 mol
= 0.724 mol
= 2.17 mol
Divide all answers
Empirical formulas
K = 1.45 mol = 2.00
0.724 mol
C = 0.724 mol = 1.00
0.724 mol
O = 2.17 mol = 3.00
0.724 mol
Therefore, the empirical formula = K2CO3
Name?
Potassium Carbonate
Molecular formulas
• Gives the type and actual number of atoms in
the compound
• CH2O – empirical formula for glucose
• C6H12O6 – molecular formula for glucose
• So far, we have been studying ionic compounds,
so molecular and empirical formulas were the
same
• In most compounds, however, not the same
Molecular formulas
• Molecular formulas can be determined
from empirical formulas
X (empirical formula) = molecular formula
(X is a whole number)
Also true . . .
Molecular molar mass = X
Empirical molar mass
Molecular formulas
Empirical formula of mercury (I) chloride is
HgCl. What is the molecular formula,
knowing that molar mass is 472.08 g/mol?
Empirical formula HgCl
Molar mass of unknown = 472.08 g/mol
1 mol Hg = 200.59 g/mol
1 mol Cl = 35.45 g/mol
Molar mass of HgCl = 236.04 g/mol
Molecular formulas
472.08 g/mol = 2.00
236.04 g/mol
2(HgCl) = Hg2Cl2