* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Dynamic insulation wikipedia , lookup
Equipartition theorem wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Calorimetry wikipedia , lookup
Internal energy wikipedia , lookup
Conservation of energy wikipedia , lookup
Thermal conductivity wikipedia , lookup
Temperature wikipedia , lookup
Heat capacity wikipedia , lookup
Heat exchanger wikipedia , lookup
First law of thermodynamics wikipedia , lookup
Heat equation wikipedia , lookup
Thermoregulation wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Thermodynamic system wikipedia , lookup
Thermal radiation wikipedia , lookup
Second law of thermodynamics wikipedia , lookup
Countercurrent exchange wikipedia , lookup
Adiabatic process wikipedia , lookup
R-value (insulation) wikipedia , lookup
Heat transfer physics wikipedia , lookup
Heat transfer wikipedia , lookup
Thermal conduction wikipedia , lookup
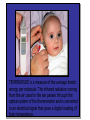
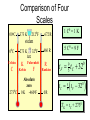
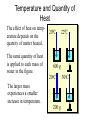
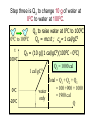
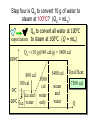
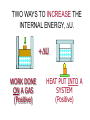
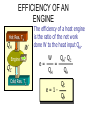
HEAT TEMPERATURE is a measure of the average kinetic energy per molecule. The infrared radiation coming from the air canal in the ear passes through the optical system of the thermometer and is converted to an electrical signal that gives a digital reading of body temperature. Temperature Temperature is related to the kinetic activity of the molecules, whereas expansion and phase changes of substances are more related to potential energy. Although not true in all cases, a good beginning is to define temperature as the average kinetic energy per molecule. T 2 ½mv N Temperature vs. Internal Energy The large pitcher and the small one have the same temperature, but they do not have the same thermal energy. A larger quantity of hot water melts more of the ice. Temperature Equilibrium Thermal Equilibrium Hot Coals Insulated Container Cool Water Same Temperature Heat is defined as the transfer of thermal energy that is due to a difference in temperature. Two objects are in thermal equilibrium if and only if they have the same temperature. Thermometer A thermometer is any device which, through marked scales, can give an indication of its own temperature. T = kX X is thermometric property: Expansion, electric resistance, light wavelength, etc. Limitations of Relative Scales The most serious problem with the Celsius and Fahrenheit scales is the existence of negative temperatures. Clearly, the average kinetic energy per molecule is NOT zero at either 00C or 00F! T = kX = 0 ? -250C ? Comparison of Four Scales 1000C 2120F 373 K 672 R 1 C0 = 1 K 460 R 5 C0 = 9 F steam 00C Celsius C -2730C 273 K ice K Kelvin 320F Fahrenheit F Absolute zero 0 K -4600F R Rankine 0R tF t 32 9 5 C tC 5 9 0 t F 320 TK = tC + 2730 Volume Expansion Expansion is the same in all directions (L, W, and H), thus: The constant b is the coefficient of volume expansion. V b V0 t Photo © Vol. 05 Photodisk/Getty FOUNDRY: It requires about 289 Joules of heat to melt one gram of steel. In this PowerPoint, we will define the quantity of heat to raise the temperature and to change the phase of a substance. Heat Defined as Energy Heat is not something an object has, but rather energy that it absorbs or gives up. The heat lost by the hot coals is equal to that gained by the water. Cool water Hot coals Thermal Equilibrium Units of Heat One calorie (1 cal) is the quantity of heat required to raise the temperature of 1 g of water by 1 C0. Example 10 calories of heat will raise the temperature of 10 g of water by 10 C0. Units of Heat (Cont.) One British Thermal Unit (1 Btu) is the quantity of heat required to raise the temperature of 1 lb of water by 1 F0. Example 10 Btu of heat will raise the temperature of 10 lb of water by 10 F0. The SI Unit of Heat Since heat is energy, the joule is the preferred unit. Then, mechanical energy and heat are measured in the same fundamental unit. Comparisons of Heat Units: 1 cal = 4.186 J 1 Btu = 778 ft lb 1 kcal = 4186 J 1 Btu = 252 cal 1 Btu = 1055 J Temperature and Quantity of Heat The effect of heat on temperature depends on the quantity of matter heated. The same quantity of heat is applied to each mass of water in the figure. 200C 220C 600 g 200C The larger mass experiences a smaller increase in temperature. 200 g 300C Quiz 1 Two objects are made of the same material, but have different masses and temperatures. If the objects are brought into thermal contact, which one will have the greater temperature change? (A) the one with the higher initial temperature (B) the one with the lower initial temperature (C) the one with the greater mass (D) the one with the smaller mass (E) the one with the higher specific heat Pre-Lecture Quiz 14 Heat Capacity The heat capacity of a substance is the heat required to raise the temperature a unit degree. Lead Glass Al Copper Iron 1000C 1000C 1000C 1000C 1000C 37 s 52 s 60 s 83 s 90 s Heat capacities based on time to heat from zero to 1000C. Which has the greatest heat capacity? Conservation of Energy Whenever there is a transfer of heat within a system, the heat lost by the warmer bodies must equal the heat gained by the cooler bodies: (Heat Losses) = (Heat Gained) Cool water Hot iron Thermal Equilibrium Change of Phase When a change of phase occurs, there is only a change in potential energy of the molecules. The temperature is constant during the change. Liquid Vaporization Solid Gas fusion Q = mLf Q = mLv Terms: Fusion, vaporization, condensation, latent heats, evaporation, freezing point, melting point. Change of Phase The latent heat of fusion (Lf) of a substance is the heat per unit mass required to change the substance from the solid to the liquid phase of its melting temperature. Q Lf m For Water: Lf = 80 cal/g = 333,000 J/kg The latent heat of vaporization (Lv) of a substance is the heat per unit mass required to change the substance from a liquid to a vapor at its boiling temperature. Q Lv m For Water: Lv = 540 cal/g = 2,256,000 J/kg Example 3: How much heat is needed to convert 10 g of ice at -200C to steam at 1000C? First, let’s review the process graphically as shown: temperature t ice steam 540 cal/g 0 100 C 1 cal/gC0 80 cal/g water 0 ice ciceand = 0.5 cal/gC only -200C ice water 00C steam and water steam only Q Example 3 (Cont.): Step one is Q1 to convert 10 g of ice at -200C to ice at 00C (no water yet). -200C 00C Q1 to raise ice to 00C: Q1 = mct t 1000C Q1 = (10 g)(0.5 cal/gC0)[0 - (-200C)] Q1 = (10 g)(0.5 cal/gC0)(20 C0) Q1 = 100 cal 00C -200C ice cice= 0.5 cal/gC0 Q Example 3 (Cont.): Step two is Q2 to convert 10 g of ice at 00C to water at 00C. Melting t 1000C Q2 to melt 10 g of ice at 00C: Q2 = mLf Q2 = (10 g)(80 cal/g) = 800 cal Q2 = 800 cal 00C -200C 80 cal/g ice and water Add this to Q1 = 100 cal: 900 cal used to this point. Q Step three is Q3 to change 10 g of water at 00C to water at 1000C. Q3 to raise water at 00C to 1000C. Q3 = mct ; cw= 1 cal/gC0 00C to 1000C t 1000C Q3 = (10 g)(1 cal/gC0)(1000C - 00C) 1 00C -200C cal/gC0 Q3 = 1000 cal Total = Q1 + Q2 + Q3 = 100 +900 + 1000 water = 1900 cal only Q Step four is Q4 to convert 10 g of water to steam at 1000C? (Q4 = mLv) Q4 to convert all water at 1000C vaporization to steam at 1000C. (Q = mLv) 1000C Q4 = (10 g)(540 cal/g) = 5400 cal 800 cal 100 cal 00C ice and -200C ice water 1000 cal water only 5400 cal Total Heat: steam and water 7300 cal Q Quiz 2 1 kg of water at 100 oC is poured into a bucket that contains 4 kg of water at 0 oC. Find the equilibrium temperature (neglect the influence of the bucket). (A) 0 oC (B) 20 oC (C) 50 oC (D) 80 oC (E) 100 oC Pre-Lecture Quiz 14 TRANSFER OF HEAT is minimized by multiple layers of beta cloth. These and other insulating materials protect spacecraft from hostile environmental conditions. (NASA) Heat Transfer by Conduction Conduction is the process by which heat energy is transferred by adjacent molecular collisions inside a material. The medium itself does not move. Conduction Direction From hot to cold. Quiz 3 Given your experience of what feels colder when you walk on it, which of the surfaces would have the highest thermal conductivity? (A) a rug (B) a steel surface (C) a concrete floor (D) has nothing to do with thermal conductivity Pre-Lecture Quiz 14 Heat Transfer by Convection Convection is the process by which heat energy is transferred by the actual mass motion of a heated fluid. Heated fluid rises and is then replaced by cooler fluid, producing convection currents. Convection is significantly affected by geometry of heated surfaces. (wall, ceiling, floor) Convection Heat Transfer by Radiation Radiation is the process by which heat energy is transferred by electromagnetic waves. Radiation Atomic No medium is required ! Sun Summary: Heat Transfer Conduction: Heat energy is transferred by adjacent molecular collisions inside a material. The medium itself does not move. Convection is the process by which heat energy is transferred by the actual mass motion of a heated fluid. Radiation is the process by which heat energy is transferred by electromagnetic waves. Examples of Thermal Conductivity Comparison of Heat Currents for Similar Conditions: L = 1 cm (0.39 in.); A = 1 m2 (10.8 ft2); t = 100 C0 2050 kJ/s 4980 Btu/h 3850 kJ/s 9360 Btu/h Concrete or Glass: 8.00 kJ/s 19.4 Btu/h Corkboard: 0.400 kJ/s 9.72 Btu/h Aluminum: Copper: THERMODYNAMICS Thermodynamics is the study of energy relationships that involve heat, mechanical work, and other aspects of energy and heat transfer. Central Heating Zeroth Law of Thermodynamics The Zeroth Law of Thermodynamics: If two objects A and B are separately in equilibrium with a third object C, then objects A and B are in thermal equilibrium with each other. Object C Thermal Equilibrium A A Object C B B Same Temperature A THERMODYNAMIC SYSTEM • A system is a closed environment in which heat transfer can take place. (For example, the gas, walls, and cylinder of an automobile engine.) Work done on gas or work done by gas INTERNAL ENERGY OF SYSTEM • The internal energy U of a system is the total of all kinds of energy possessed by the particles that make up the system. Usually the internal energy consists of the sum of the potential and kinetic energies of the working gas molecules. Quiz 4 The water flowing over Niagara Falls drops a distance of 50 m. Assuming that all the gravitational energy is converted to thermal energy, by what temperature does the water rise? (A) 0.10 C° c water 4186 J kgCo (B) 0.12 C° (C) 0.37 C° (D) 0.42 C° P.E.=mgh Joule = newton/meter Heat 14 (13 of 42) TWO WAYS TO INCREASE THE INTERNAL ENERGY, U. +U WORK DONE ON A GAS (Positive) HEAT PUT INTO A SYSTEM (Positive) TWO WAYS TO DECREASE THE INTERNAL ENERGY, U. Wout Qout -U Decrease hot WORK DONE BY EXPANDING GAS: W is positive hot HEAT LEAVES A SYSTEM Q is negative THE FIRST LAW OF THERMODYAMICS: • The net heat put into a system is equal to the change in internal energy of the system plus the work done BY the system. Q = U + W final - initial) • Conversely, the work done ON a system is equal to the change in internal energy plus the heat lost in the process. HEAT ENGINES Hot Res. TH Qhot Engine Qcold Cold Res. TC Wout A heat engine is any device which through a cyclic process: • Absorbs heat Qhot • Performs work Wout • Rejects heat Qcold THE SECOND LAW OF THERMODYNAMICS Hot Res. TH Qhot Engine Wout Qcold Cold Res. TC It is impossible to construct an engine that, operating in a cycle, produces no effect other than the extraction of heat from a reservoir and the performance of an equivalent amount of work. Not only can you not win (1st law); you can’t even break even (2nd law)! The Second Law of Thermodynamics The second law of thermodynamics is a statement about which processes occur and which do not. There are many ways to state the second law; here is one: Heat will flow spontaneously from a hot object to a cold object. It will not flow spontaneously from a cold object to a hot object. EFFICIENCY OF AN ENGINE Hot Res. TH QH W Engine QC The efficiency of a heat engine is the ratio of the net work done W to the heat input QH. e= W QH = Cold Res. TC e=1- QH- QC QH QC QH REFRIGERATORS Hot Res. TH Qhot Win Engine Qcold Cold Res. TC A refrigerator is an engine operating in reverse: Work is done on gas extracting heat from cold reservoir and depositing heat into hot reservoir. Win + Qcold = Qhot WIN = Qhot - Qcold Entropy Entropy Entropy is a measure of the disorder of a system. This gives us yet another statement of the second law: Natural processes tend to move toward a state of greater disorder. Example: If you put milk and sugar in your coffee and stir it, you wind up with coffee that is uniformly milky and sweet. No amount of stirring will get the milk and sugar to come back out of solution. Entropy Another example: when a tornado hits a building, there is major damage. You never see a tornado approach a pile of rubble and leave a building behind when it passes. Another consequence of the second law: In any natural process, some energy becomes unavailable to do useful work.