* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Basic Physical Chemistry (12.4 MB ppt)
Rutherford backscattering spectrometry wikipedia , lookup
Chemical potential wikipedia , lookup
Heat transfer physics wikipedia , lookup
Rate equation wikipedia , lookup
Reaction progress kinetic analysis wikipedia , lookup
Industrial catalysts wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Marcus theory wikipedia , lookup
Thermodynamics wikipedia , lookup
Electrochemistry wikipedia , lookup
Enzyme catalysis wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Chemical equilibrium wikipedia , lookup
George S. Hammond wikipedia , lookup
Equilibrium chemistry wikipedia , lookup
Physical organic chemistry wikipedia , lookup
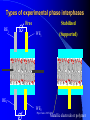
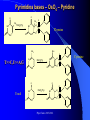
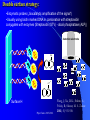
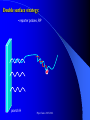
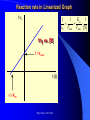
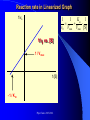
The texts were not checked by a native speaker. All comments, suggestions and improvements are welcome and the authors will be very thankful for discovered errors, advices, recommendations and remarks. We are waiting for your messages on E-mail address: [email protected] Physical Chemistry in Biochemistry Basic Medical Chemistry and Biochemistry 1st year © Institute of Medical Biochemistry and Laboratory Diagnostics of the General University Hospital and of The First Faculty of Medicine of Charles University in Prague - 2005-2016 Sylabus of the Lecture The most important chapters of physical chemistry Thermodynamics, Thermochemistry, Chemical equilibrium, Kinetics (Reaction rate, order of chemical reaction), Catalysis Photos and films connected with history of Electrochemistry J. Heyrovsky - Nobel Prize for polarography 1959 J. Heyrovsky – Inventor of polarography 1959 Promotion clip on polarography Oscilopolarography (Expo 58 - Brussels) Promotion film of J. Heyrovský Institute of Physical Chemistry of the Academy of Sciences of the Czech Republic, v.v.i., 2007 Promotion film of J. Heyrovský Institute of Physical Chemistry of the Academy of Sciences of the Czech Republic, v.v.i., 2009 Phys.Chem. 2015/2016 Physical Chemistry in Biochemistry The Most Important Topics Physical chemistry deals with application of physics to macroscopic, microscopic, atomic, subatomic, and particulate phenomena in chemical systems within the field of chemistry traditionally using the principles, practices and concepts of thermodynamics, quantum chemistry, statistical mechanics and kinetics. It is mostly defined as a large field of chemistry, in which several sub-concepts are applied; the inclusion of quantum mechanics is used to illustrate the application of physical chemistry to atomic and particulate chemical interaction or experimentation. http://en.wikipedia.org/ Phys.Chem. 2015/2016 Research Institutes dealing with physical chemistry and biochemistry J. Heyrovský Institute of Physical Chemistry of the Academy of Sciences of the Czech Republic, v.v.i. Institute of Biophysics of the Academy of Sciences of the Czech Republic, v.v.i. Faculty of Science, Charles University in Prague Faculty of Science, Masaryk University in Brno Czech University of Life Sciences, Prague Institute of Chemical Technology, Prague Faculty of Chemical Technology, Pardubice … Phys.Chem. 2015/2016 Terminology Physical chemistry Chemical physics Biophysical chemistry … ???Borders??? Application of Physical Chemistry User (physician) does not use the principle of the applied method Temperature measurement – linear thermal expansion of liquids (mercury, ethanol), thermocouple - Physics Sedimentation – gravitation - Physics Centrifugation – centrifugal power - Physics Utilization of results only Phys.Chem. 2015/2016 Some of the most Important Topics of Physical Chemistry applied in Biochemistry Thermodynamics Thermochemistry Chemical equilibrium Kinetics (Reaction rate, order of chemical reaction) Catalysis … Phys.Chem. 2015/2016 Thermodynamic laws Thermodynamics – science dealing with energy transports by physical and chemical processes (shortened version) - the study of the conversion of heat energy into different forms of energy (in particular, mechanical, chemical, and electrical energy); different energy conversions into heat energy; and its relation to macroscopic variables such as temperature, pressure, and volume. Its underpinnings, based upon statistical predictions of the collective motion of particles from their microscopic behavior, is the field of statistical thermodynamics, a branch of statistical mechanics (full definition) Phys.Chem. 2015/2016 Thermodynamic laws Closed system – does not exchange either mass or energy (E = mc2) Open system – exchanges mass and/or energy vs. Closed system – does not exchange mass; exchanges energy only Open system – exchanges mass and/or energy Isolated system – does not exchange either mass or energy Vs. Isolated Systems – matter and energy may not cross the boundary Adiabatic Systems – heat must not cross the boundary Diathermic Systems - heat may cross boundary Closed Systems – matter may not cross the boundary Open Systems – heat, work, and matter may cross the boundary (often called a control volume in this case) Phys.Chem. 2015/2016 Thermodynamic laws P - pressure Internal energy U Helmholtz free energy A=U-TS T – Temperature Enthalpy H=U+pV E – potential Gibbs free energy G=H-TS a – activity V - Volume Electrochemical potential m=m0-RT ln (a) - zFE F – Farraday constant First Law of Thermodynamics Sum of all energies in closed system is constant, irrespective of running physical or chemical processes – work is changed into energy and energy into work (In a closed system (see below) the total inflow of energy must equal the total outflow of energy. dU = dw + dq (correctly should be , not d, it is not the total differential) U - internal energy, w - work, q – heat Phys.Chem. 2015/2016 Thermodynamic laws II Second law of thermodynamics dQ dS T Q S T S…entropy (measure of disorderliness of the system – with increasing inordinance of the system increases its entropy); Q…heat, T…temperature The total entropy of any isolated thermodynamic system tends to increase over time, approaching a maximum value. The heat cannot spontaneously pass from the colder body to the warmer one. The entropy of an isolated system is constant or increasing. It is not possible to construct the periodically working machine, which would utilize the heat from one accumulator only and which would perform the work exactly equivalent to this heat. It is not possible to construct perpetum mobile of the second type. All spontaneous processes are realized with increasing entropy, with increasing disorderliness of the system. Phys.Chem. 2015/2016 Thermodynamic laws III Third law of thermodynamics lim S 0 T 0 - the entropy of all systems and of all states of a system is zero at absolute zero - it is impossible to reach the absolute zero of temperature by any finite number of processes Energy types 1. Free energy – „noble“, it can be free transported, transformed (chemical, electric) 2. Bound energy – heat, which can be transported (flow) only in the direction of the heat gradient. Transformation to other types of energy can be realized only, when the warmer body gives its energy to the colder one. Phys.Chem. 2015/2016 Gibbs energy dG SdT VdP Ad ... m~i dni i H… Enthalpy H = U+pV (increase of enthalpy is equal to the heat, which the system gains under constant pressure, and at the same time no any other then volume work is produced) G … Gibbs energy G = H-TS (= Maximal reversible work other then volume work, which the system gains (produces) by constant temperature and pressure γ … surface tension A … area ~… m m electrochemical potential G ~ m i ni T , P , i ,ni j Negatively taken work necessary for releasing of 1 mol of charged particles and their transport into infinitively diluted state m~ m0 RT ln a zFE Phys.Chem. 2015/2016 Thermodynamic laws VI - Terminology Reversible: system is passing through huge amount of small state changes, by which it is always in equilibrium with surroundings; in any moment it is possible to stop it and to change the direction of the process Irreversible: all changes, which differs from the reversible process Isobaric: P = const. (pressure) Isothermic: T = const. (temperature) Isochoric: V = const. (volume) Adiabatic: q = const. (heat) Phys.Chem. 2015/2016 Exothermic reaction : ΔH < 0 Endothermic reaction : ΔH > 0 Spontaneous reaction: ΔG < 0 (ΔG= ΔH-TΔS) Unspontaneous reaction: ΔG> 0 Equilibrium ΔG = 0 Thermochemistry Reaction heat: heat, which the system gains (released), if under constant pressure the chemical reaction in extension of 1 mol is realized according to the given equation, provided that the temperature of the system before the reaction is the same as after the reaction and that reactants as well as products are in the phase given in reaction equation. C(s)+O2(g)=CO2(g) Phys.Chem. 2015/2016 Thermochemical laws First thermochemical law (Lavoisier-LaPlace’s) The total heat released by the chemical reaction is equal to that one, consumed by the reversed direction of the reaction. A ↔B;ΔHA→B = -ΔHB→A Second thermochemical law (Hess’s) If a chemical reaction is realized in a few sequential steps, the sum of the energy, released (consumed) in single steps, is equal to the total energy, which would be released (consumed), if the reaction would be realized directly in the only one step. A→ B → C; ΔHA→C = ΔHA→B + ΔHB→C It enables to determine the caloric value of foods by their burning up, although in human body are they metabolized in plenty of gradual steps (glucose, lipids etc.). Calculation of reaction heat is realized from combination heats (heat, which is released (consumed) by formation o 1 mol of the compound directly from atoms under constant pressure and temperature) or from combustion heats (heat, which is released, by combustion of 1 mol of compound in pure oxygen by formation of most stable oxidizing Phys.Chem. 2015/2016 products). Heat exchange 1. Without any change of the state T2 H Q c p dT c p (T2 T 1) i T1 Q m.c p (T2 T1 ) T2 c p C p dT A B(T2 T 1) T1 C (T2 T 1) 2 2 2. Latent heat HVaporization, Solidification, Liquefaction, Fusion 3. Thermochemical law Q1 Q2 Heat of fusion = Latent heat of solidification Latent heat of vaporization = latent heat of condensation Phys.Chem. 2015/2016 Heat exchange - 1 kg of water 1. heating from 0 oC to 100 oC Q m.c p (T2 T1 ) 1* 4.2 *100 kJ 420 kJ 2. Heat of vaporization (normal boiling point) HVaporization 2256kJ ~ heating from 0 to 540 oC 3. Melting point (normal fusion point) H Tání 333.7kJ Phys.Chem. 2015/2016 ~ heating from 0 to 80 oC Chemical equilibrium A B C D Reactants A and B; Products C and D In equilibrium state runs the reaction from the left side to the right side by the same rate K Guldberg-Waage’s law (1863) C D AB K ... equilibrium constant of the reaction K ... depends on T, P etc. In words: The product of molar concentrations of products of the reaction divided by the product of molar concentrations of reactants is in equilibrium state constant in closed system. Le Chatelier's principle If a chemical system at equilibrium experiences a change in concentration, temperature, volume, or partial pressure, then the equilibrium shifts to counter-act the imposed change. Phys.Chem. 2015/2016 Chemical equilibrium II [C].[D] = [B].[A]=> K=1 [C].[D] > [B].[A] => K>1 – prevailing products [C].[D] < [B].[A] => K<1 –prevailing reactants Oscillating reactions: e.g., Zhabotinsky To influence the course of the reversible reaction (its direction), it is necessary to work in open system. If one component of the reaction is removed, the system produces the removed amount continuously to reach (restore) the equilibrium. Thereby we can reach practically total realization of the reaction in the direction, in which it practically does not run in closed system (K<<1). Phys.Chem. 2015/2016 Possibilities of influencing of steady state (equilibrium) C D 1. Decrease of final products quantity K 2. Increase of starting compounds quantity AB 3. By inequal number of molls of starting compounds and final product in gaseous system (2A + B = C) the change of pressure 4. Change of temperature (exothermic – the rate decreases with increasing temperature; endothermic – the rate increases with increasing temperature) All reactions in living systems are realized in open systems, consequential, consecutive reaction takes off products of previous reaction, whereby the equilibrium (steady) state is disturbed and so influences the course of the reaction. A+B=C+D → D+E=F+G → G+H=I+J → J+K=L+M The compound M is as the final product of the metabolism removed from the (living) system away, e.g., by respiration or excretion. Catalyzers influence the reaction rate, but not the equilibrium. They are enabling other reaction way, energetic of the reaction, but not the Phys.Chem. 2015/2016 equilibrium!!! Reaction rate, order of chemical reaction d [ A] d [C ] v k C dt dt AC A B C D v k C D v K k C D k AB v k AB Equilibrium: 1st order reaction 2nd order reaction v v k A AC D 2 v k A 2A C D v k AB A B C D Reaction of more than 2nd order is realized in fact stepwise, gradually, as the reaction composed of more reaction substeps. For the reaction rate is the controlling the slowest one). Phys.Chem. 2015/2016 Influence of the Temperature on the Reaction Rate Increase of the temperature increases the reaction rate. Their relationship is given by Arhenius equation: E k A. exp a RT k... rate constant, A… function factor; T … absolute temperature; Ea… activation energy; R universal gas constant It follows that the increase of the temperature essentially increases the reaction rate – exponentially. activity This fact is commonly used by homonotermn organisms, e.g., by defense reactions, such reactions run at higher temperature faster and they are more effective. Coeffitient Q10 = how many times changes the reaction rate by the change of the temperature by 10 grades ~ 2 Phys.Chem. 2015/2016 Electric doublelayer Electrode Electrode Diffusion part Helmholtz part Use: a) Electrolysis of the solutions b) Electroplating c) Tooth cell – improper materials d) Voltammetry e) Power sources Phys.Chem. 2015/2016 Solution Oxidation - reduction reactions (redox) Example Galvanic cell - spontaneous Anode: Zn=Zn2++2e1st redox system E0(Zn2+/Zn)=-0.76 V Cathode: Cu2++2e-=Cu 2nd redox system E0(Cu2+/Cu)=0.34 V Zn(s) + Cu2+ = Cu(s) + Zn2+ U=Ec-Ea Measurement of redox potential (Secondary school) Electrolytic cell – Inserted voltage Anode: Cu = Cu2+ + 2e1st redox system Cathode: Zn2+ + 2e- = Zn 2nd redox system Cu(s) + Zn2+ = Zn(s) + Cu2+ U=Ea-Ec Reduction is always realized at cathode! E1 E10 a a RT RT RT a1red ln U E1 E 2 E10 - E02 ln 1red ln 2red nF a1ox nF a1ox nF a 2ox Phys.Chem. 2015/2016 Oxidation - reduction reactions (redox) Redox pair [V] Redox pair [V] Li+/Li (s) - 3.04 Co2+/Co (s) - 0.28 K+/K (s) -2.92 Ni2+/Ni (s) - 0.25 Na+/Na (s) - 2.71 Sn2+/Sn (s) - 0.14 Ca2+/Ca (s) -2.50 Pb2+/Pb (s) - 0.13 Al3+/Al (s) - 1.66 2H+/H2 (g) +0.00 Mn2+/Mn (s) - 1.18 Sn4+/Sn2+ +0.15 Zn2+/Zn (s) - 0.76 Cu2+/Cu (s) +0.34 Cr3+/Cr (s) - 0.74 Ag+/Ag (s) +0.80 Fe2+/Fe (s) - 0.44 Cl2/2Cl-(g) +1.36 Cd2+/Cd (s) - 0.40 Au+/Au (s) +1.50 Tl+/Tl (s) - 0.34 Phys.Chem. 2015/2016 Oxidation - reduction reactions (redox) I Standard electrode potentials at 25 oC in aqueous solutions Phys.Chem. 2015/2016 Oxidation - reduction reactions (redox) II The redox pair with the higher standard potential is the oxidant of the redox pair with the lower standard potential. Voltage change: 1. Connection of two different metals 2. Connection of the same metals dipped in two different electrolytes (different concentration) Electric current direction a) Galvanic cell b) electrolytic cell Phys.Chem. 2015/2016 Oxidation - reduction reactions (redox) III Comparison of efficiency of energy production by microorganisms. In parentheses are numbers corresponding to ΔGO’ in kJ.mol-3, cytFe3+ and cytFe2+ are oxidized and reduced forms of cytochroms Phys.Chem. 2015/2016 Redox reactions in living organism Living organisms commonly use redox reactions as energy sources. A number of organic compounds exist in oxidized form as well as in reduced form and therefore they can be involved in the transport of electrons. In these processes the organisms gain the energy necessary for life. Transferred electrons enable, e.g., transport of protons (H+) through membranes and enable the changes of pH. Accumulated protons by reverse transport through the membrane can supply the energy for the transport of other compounds or for the synthesis of ATP. From redox potential of two equal redox systems we can calculate ΔGo of the chemical reaction ΔGo = -zF ΔE’o (z – number of transported electrons) by the change of the redox potential ΔE’o (ΔE’o - „biologic“ standard reduction potential – standard system state for pH=7) [Ared]=[Aox]. Phys.Chem. 2015/2016 Redox reactions in living organism II During the aerobe transformation of compounds (metabolism) is realized (in principle) strong exergonic reaction (and exothermic) redox reaction: 2H2+O2→2H2O. The high energetic electrons of hydrogen are transported on oxygen in many sequential steps. Their energy is used for living processes of the cell (organism). These processes are studied in biochemistry. As electron carrier are often used metals bound on peptides. Spontaneous reaction = exergonic (exothermic) Non-spontaneous reaction = endergonic (endothermic) 2H2(g)+O2(g)→2H2O(g) ΔGo =-242 kJ.mol-1 – exothermic (burning) 2H2O(g) → 2H2(g)+O2(g) ΔGo =242 kJ.mol-1 – endothermic, equilibrium is shifted to the left, only by high temperature and decreased pressure starts the decomposition (at 2100 oC and 0.1 MPa reacts 2 % of molecules only) . Phys.Chem. 2015/2016 Scheme of the Polarographic Device W - Working electrode: Polarography – dropping (mercury) electrode Voltammetry – stationary surface - Hanging mercury drop electrode Solid2015/2016 electrode Phys.Chem. First polarograms Phys.Chem. 2015/2016 First paper on polarography – 1922 in journal “Chemicke listy” Phys.Chem. 2015/2016 December 10th, 1959 (37 years after discovery of polarography) was awarded Prof. Jaroslav Heyrovský by the Swedish king Gustavo Adolph VI. in Stockholm by Nobel prize for chemistry Phys.Chem. 2015/2016 Phys.Chem. 2015/2016 Order for production of the first polarograph and one of the first polarographic machines Phys.Chem. 2015/2016 Mercury fountain (Spain) Barcelona, Foundation Miró Mercury mine Almaden Phys.Chem. 2015/2016 Modern polarographic device – small amount of mercury (produced in Czech republic 21st century) Phys.Chem. 2015/2016 Inorganic analysis H Li Be B C N O F Na Mg Al Si P S Cl K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Rb Sr Y Zr Nb Mo Te Ru Rh Pd Ag Cd In Sn Sb Te I Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Fr Ra Ac Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr (Elements determined by DC polarography are in full lined boxes, by ASV in double boxes, and by AdSV are underlined ) Phys.Chem. 2015/2016 Applications of polarography (voltammetry) Metals (cations): Cd, Cu, Zn, Pb, Sn, Tl, Mo, Cr, Ni, Ag, V, Hg, …Speciation (different valences!!!) Anions: Chlorides, bromides, iodides, iodates, sulphates, phosphates, nitrates, nitrites Organic compounds: Amino acids (cysteine, cystine,…) Glutathione, metallothioneins Nukleic acids (adenine, guanine, thymine, cytosine, uracil) Nitro compounds (TNT, nitrobenzene) Thiodiglycolic acid Phenylglyoxylic acid Carcinogens, toxic compounds, medicals, pesticides … Phys.Chem. 2015/2016 Main fields of application of polarography (voltammetry) today 1. Mechanistic studies esp. of organic substances - importance for basic research structure activity relationship clue for biological processes supramolecular interactions electrosynthesis electroanalysis Phys.Chem. 2015/2016 Main fields of application of polarography (voltammetry) today Trace metal and inorganic determination and speciation bioavailability studies water analysis soil analysis 3. Trace organic analysis Pharmaceuticals Preparations Biological samples Metabolites Herbicides and pesticides Explosives Ecotoxic substances Dyes Chemical carcinogens 2. Phys.Chem. 2015/2016 LDR of various polarographic and voltammetric techniques Phys.Chem. 2015/2016 Phospholipid bilayers Real Model Aqueous Outer area Hydrophillic part Phospholipid bilayer Hodrophobic part Aqueous Inner area Phys.Chem. 2015/2016 Types of experimental phase interphases Free RE1 Stabilized WE1 (Supported) RE2 WE2 Phys.Chem. 2015/2016 Metallic electrode or polymer Patch Clamp Technique Classic P-C technique Planar P-C technique Phys.Chem. 2015/2016 DNA Adenine: • 6-electron reduction including deamination • Under normal conditions 4electron Cytosine: • 3-electron reduction (deamination + dimerization) Phys.Chem. 2015/2016 OSCILOGRAFIC POLAROGRAPHY Controlled insertion of auxiliary current dE/dt dsDNA ssDNA CA Cathodic part Poorly soluble compound with Hg Anodic part G E J.N.Davidson and E.Chargraff: The Nucleic Acids, Vol.1, Academic Press, New York 1955 Palecek E.: Oszillographiche Polarographie der Nucleinsauren und ihrer Bestandteile; Naturwiss. 45 (1958) 186 Palecek E.: Oscillographic polarography of highly polymerized deoxyribonucleic acid; Nature 188 (1960) 656 Phys.Chem. 2015/2016 Various techniques of analysis ADSORPTIVE STRIPPING ADSORPTIVE TRANSFER STRIPPING Nucleic acid in the cell with electrolyte is accumulated at the electrode surface Nucleic acid is accumulated at the small electrode from very small drop of the sample (3-10 ml) Phys.Chem. 2015/2016 The sample (nucleic acid) is transferred into the pure eelctro;yte Cytosine on Silver solid amalgam electrode m-AgSAE 2.10-5 M; 0,1 M acetate buffer; pH 4,8 -1600 Adenin - Rce 31 (Id=Id; Ic=0; Ik=0; Iir=0) -600 -1000 -100 -1100 -1200 -1300 -1400 -1500 -18000 400 Rce 23 (Id=Id; Ic=0; Ik=0) E [mV] -12000 adenin 1.10-4 M + cytosin 1.10-4 M v=80-160-320-640 mV.s-1; I [nA] I [nA] -1100 I ref - 20-40-80-160 20-40-80-160 I ref - 40-80-160-320 40-80-160-320 I ref - 80-160-160-320 80-160-160-320 -6000 rce (23) 0 -1100 -1300 -1500 -1700 E [mV] Phys.Chem. 2015/2016 Mixture of adenine a guanine -800 blank Ade Gua I [nA] -600 mixture adenine : guanine 58 : 42 -400 -200 0 -700 -900 E [mV] -1100 adenine guanine CT DNA p DNA Critical level nmol.L-1 0.67 0.82 0.86 0.75 Limit of detection nmol.L-1 0.95 1.85 1.75 1.25 Limit of determination nmol.L-1 3.06 6.70 5.99 6.93 RSD % 1.28 1.83 0.47 0.57 Phys.Chem. 2015/2016 CT - Calf thymus DNA; p DNA - plazmidová DNA; C. Interaktion of damaged (denaturated) DNA with osmium complexex OsO4 (L) O + N -O Os L = Pyridine -O 1,10–Fenantroline Bipyridyl Phys.Chem. 2015/2016 O + N Pyrimidina bases – OsO4 – Pyridine O O CH3 NH O O CH3 OsO4(Py) O NH O NH + N Os NH O O Thymine + N NH2 NH2 N T>>C,U>>A,G O O O NH Uracil O O + O N O N O OsO4(Py) NH Os NH O NH Cytosine O N OsO4(Py) + N O NH O + Os NH Phys.Chem. 2015/2016 O O + N DNA modiffied with Os(py) on HMDE a m-AgSAE DNA 2 mg.l-1; 0,1 M acetate buffer; pH 4,8 -5500 20 mV/s 40 mV/s 80 mV/s 160 mV/s 320 mV/s 640 mV/s -4500 i [nA] -3500 -2500 -1500 -500 -1200 -1250 -1300 -1350 -1400 -1450 -1500 -1500 E [mV] -1300 2) A- +H+ HAAds -1100 i [nA] 1) HAAds+e-A-+Hads -900 -700 -1550 -1600 Os-DNA_A2 20 mV/s 40 mV/s 80 mV/s 160 mV/s 320 mV/s 640 mV/s -500 -300 -100 -900 -1000 Phys.Chem. 2015/2016 -1100 -1200 -1300 E [mV] -1400 -1500 -1600 Hybridization of DNA (RNA) is based on the principle of double helix formation from two complementary strands hybridization probe CGAATACGACCTTA Sequence of the probe is arranged (synthetized) with respect to the DNA sequence, which is CGAATACGACCTTA GCTTATGCTGGAAT GCTTATGCTGGAAT Target DNA is detected using the probe Phys.Chem. 2015/2016 Hybridization of DNA (RNA) is based on the principle of double helix formation from two complementary strands This principle is used in various moidification in routine analysis: • Detection of some nucleotide sequences • Detection of mutations, „polymorphisms“ in some sequences of genome • Determination of gen expressions Phys.Chem. 2015/2016 From practical reason is one end of the probe connected to the solid surface Immobilized probe is exposed to the analyzed DNA (RNA) sample If the sample contains the chain of DNA (RNA) with complementary sequence to the probe („target sequence “), the double helix („duplex“, „hybrid“) is formed on the solid surface Phys.Chem. 2015/2016 From practical reason is one end of the probe connected to the solid surface If the sample contains the chain of DNA (RNA) with complementary sequence to the probe („target sequence “), the double helix („duplex“, „hybrid“) is formed on the solid surface Phys.Chem. 2015/2016 Non-specific DNA molecules are removed (washed off) If the sample contains the chain of DNA (RNA) with complementary sequence to the probe („target sequence “), the double helix („duplex“, „hybrid“) is formed on the solid surface Phys.Chem. 2015/2016 Non-specific DNA molecules are removed (washed off) The detection step follows It is advantageous to mark DNA with some detectable sensor (radionuclide, fluoroform…) Phys.Chem. 2015/2016 DNA („arrays“): • Simultaneous application of a lot of probes • Application of different (of different “colors“) fluorescence probes • Commercial available devices (Affymetrix…) Phys.Chem. 2015/2016 Electrochemical sensor for DNA hybridization: electrode with hybridization probe on the surface I hybrid samotná sonda Phys.Chem. 2015/2016 E Double surface strategy: • Hybridization is realized on one surface (H), which was optimized for these purposes; it is not necessary to be an electrode • separation of target DNA • target DNA from the surface H is released and electrochemically determined Detection electrode surface H Phys.Chem. 2015/2016 Double surface strategy: : • Hybridization is realized on one surface (H), which was optimized for these purposes; it is not necessary to be an electrode • separation of target DNA • target DNA from the surface H is released and electrochemically determined detekční elektroda Phys.Chem. 2015/2016 Double surface strategy: the use of magnetic beads Surface H Detection electrode detection cílová DNA Nonspecific DNA magnetic beads with hybridization probe ~1µm hybridization separation magnet Phys.Chem. 2015/2016 Release of target DNA Double surface strategy: •Enzymatic probes („biocatalytic amplification of the signal“) •Usually using biotin marked DNA in combination with streptavidin conjugates with enzymes (Streptavidin (STV) - alcali phosphatase (ALP)) detection electrode OH OH OH OH OH OH OH OH OH OH OH OH P OH OH O OH Surface H Phys.Chem. 2015/2016 Wang, J.; Xu, D. K.; Erdem, A.; Polsky, R.; Salazar, M. A. Talanta 2002, 56, 931-938. O P alkalic phosphatase OH Palecek, E.; Billova, S.; Havran, L.; Kizek, R.; Miculkova, A.; Jelen, F. Talanta 2002, 56, 919-930. Phys.Chem. 2015/2016 Double surface strategy: • reporter probes, RP povrch H Phys.Chem. 2015/2016 Catalysis Catalysis is the process, in which the rate (not equilibrium) of a chemical reaction is increased (decreased, respectively) by means of a chemical substance known as a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed. The catalyst may participate in multiple chemical transformations, although in practice catalysts are secondary processes. it changes the reaction mechanism, it changes the activation energy, it is involved in the formation of the activation complex A+B→AB vs. A+B+K→ABK→AB+K EAB EAB – activation energy without catalysis EABK – activation energy with catalysis GAB – Gibbs energy of the reaction Phys.Chem. 2015/2016 EABK GAB products Example: Catalytic production of sulphuric acid Sulfuric acid is produced from sulfur, oxygen and water via the contact process. In the first step, sulfur is burned to produce sulfur dioxide. (1) S(s) + O2(g) → SO2(g) This is then oxidised to sulfur trioxide using oxygen in the presence of a vanadium(V) oxide catalyst. (2) 2 SO2 + O2(g) → 2 SO3(g) (in presence of V2O5) Finally the sulfur trioxide is treated with water (usually as 97-98% H2SO4 containing 2-3% water) to produce 98-99% sulfuric acid. (3) SO3(g) + H2O(l) → H2SO4(l) Phys.Chem. 2015/2016 Reaction rate E S ES k1 , k 1 k 1 k 2 Km k1 k2 E P Michaelis constant Km dP [S] vo Vmax dt [S] K m Michaelis–Menten equation The rate of production of the product, is referred to as the reaction rate, V in enzyme kinetics. Vmax = maximal rate for the given catalyst concentration (Double) Reciprocal expression K 1 1 1 m v0 Vmax Vmax [S] Phys.Chem. 2015/2016 Graphical expression of Michaelis–Menten equation 1st order kinetic vo [S] K m vo Vmax 0 order kinetic Vmax Vmax 2 vo Vmax [S] [S] V [S] Vmax K max v2o [S] [S] 2[Sm] Vmax [S] [S] Vmax Vmax k[S]0 [S] K m [S] [S] [S] Vmax Vmax [S] [S] 2[S] 2 Area of catalyst enzym nasycen saturation by a substrátem substrate [S] mol/l Km Phys.Chem. 2015/2016 Reaction rate in Linearized Graph 1/vo Km 1 1 1 v0 Vmax Vmax [S] 1/v0 vs. [S] 1 / V m ax 1/[S] - 1 / Km Phys.Chem. 2015/2016 Reaction rate in Linearized Graph 1/vo Km 1 1 1 v0 Vmax Vmax [S] 1/v0 vs. [S] 1 / V m ax 1/[S] - 1 / Km Phys.Chem. 2015/2016 Competitive inhibition Some molecules inhibit catalysis by competing for the active sites. The strongest inhibitors are called poisons. CH3OH HCOOH C2H5OH CH3COOH Alcohol dehydrogenase The catalyst is competitively inhibited by a non-toxic substrate to the prejudice of toxic substrate The maximal rate is reached at higher [S] values Vmax is not changed Km is increased Phys.Chem. 2015/2016 Competitive inhibition Vmax 1/vo vo 1 / Vmax 1 / Vmax [S]-1 Km Km inh [S] mol.l-1 - 1/Km - 1/Km Phys.Chem. 2015/2016 Non-competitive inhibition The inhibitor (substrate) binds to the enzyme at a site other than the catalyst's active site (this other site is called an allosteric site). Km is not changed (active site is free for the substrate) Vmax is decreased, because the concentration of E-S complex decreases In this mode of inhibition, there is no competition between the inhibitor and the substrate, so increasing the concentration of the substrate still does not allow the maximum enzyme activity rate to be reached. Phys.Chem. 2015/2016 Non-competitive inhibition v0 Vmax 1/vo Vmax inh 1 / Vmax inh 1 / Vmax 1/[S] Km Km inh [S] mol.l-1 - 1 / Km - 1 / Km Phys.Chem. 2015/2016 Research topics in Physical Chemistry www.jh-inst.cas.cz • Development of new fluorescence methods and their application in the research of structure, functionality and dynamics of biomembranes; single molecule spectroscopy in biological systems, dynamics characterization in model and biomembranes on the picosecond to millisecond time scale; characterisation of DNA condensation processes relevant to gene therapy; advanced in vivo fluorescence microscopy. • Elucidation of the function of biologically active molecules based on their electrochemical reactivity. The interim aims will be the elucidation of (i) creation and stability of monolayers on polarized interfaces and (ii) charge transfer reactions. • preparation and characterization of phospholipid bilayers at stabilized phase boundaries enabling studies of transfer of charged and uncharged species between the two phases • studies of transfer of charged and uncharged species between the two phases • transport of compounds across the model membrane using cell permeable peptides • studies on electrochemical properties of biologically important species • development of new types of electrochemical sensors, devices and methods for following DNA, proteins and other biologically active substances; • novel techniques of DNA damage detection; Phys.Chem. 2015/2016 Research topics in Physical Chemistry www.jh-inst.cas.cz • research on function and structure of metallothioneins and phytochelatins (bioligands); • biophysical and biochemical problems accompanying utilization of electrochemical techniques; • state of solutions and its effect on electrode processes; • bioavailable forms of trace elements present in soil solution and their availability to plants. • Molecular sieve chemistry and catalysis • Synthesis of zeolites, zeotypes, mesoporous molecular sieves and • hierarchic materials combining micro and meso porosity • Design and characterization of new photosensitisers and investigation of their interactions with target biological macromolecules Phys.Chem. 2015/2016