* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Dynamic insulation wikipedia , lookup
Thermoregulation wikipedia , lookup
Heat exchanger wikipedia , lookup
Solar air conditioning wikipedia , lookup
Radiator (engine cooling) wikipedia , lookup
R-value (insulation) wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Heat equation wikipedia , lookup
Cogeneration wikipedia , lookup
Thermal conduction wikipedia , lookup
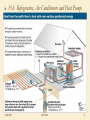
15.1 The First Law of Thermodynamics A system’s internal energy can be changed by doing work or by the addition/removal of heat: ΔU = Q - W W is negative if work is done on the system What is the state of the system? 5/23/2017 Compression of the gas Described by P, V, T, m, U APHY201 1 15.2 Thermodynamic Processes and the First Law Isothermal: T = constant → ΔU = 0 → W = Q Adiabatic: Q = 0 → ΔU = -W 5/23/2017 APHY201 2 15.2 Thermodynamic Processes and the First Law 5/23/2017 If pressure is constant then W = Fd = PAd = P ΔV APHY201 3 15.2 Thermodynamic Processes and the First Law 5/23/2017 The total work done during a process is equal to the area under the PV diagram APHY201 4 15.4 The Second Law of Thermodynamics Heat can flow spontaneously only from a hot object to a cold object. A reversible process is one that is always in equilibrium and can return to its initial conditions along the same path Most natural processes are irreversible Sets an upper limit on efficiency of heat engines 5/23/2017 APHY201 5 15.5 Heat Engines Heat engines convert U into other useful forms of energy – mechanical, electrical, … ΔUcycle = 0 → QH = W + QL Automobile engines 5/23/2017 APHY201 6 15.5 Heat Engines W QL 1 The efficiency of a heat engine is e QH QH Carnot (ideal) engine Reversible processes Too slow for real engines 5/23/2017 APHY201 7 15.6 Refrigerators, Air Conditioners and Heat Pumps A heat engine in reverse. QL COP W 5/23/2017 APHY201 8 15.6 Refrigerators, Air Conditioners and Heat Pumps 5/23/2017 APHY201 9 2. (a) The work done by a gas at constant pressure is found from Eq. 15-3. 1.01105 Pa 3 3 5 W PV 1 atm 18.2 m 12.0 m 6.262 10 J 1 atm (b) The change in internal energy is calculated from the first law of thermodynamics 4186 J 5 6 U Q W 1400 kcal 6.262 10 J 5.2 10 J 1 kcal 5/23/2017 APHY201 10 26. Find the exhaust temperature from the original Carnot efficiency, and then recalculate the intake temperature for the new Carnot efficiency, using the same exhaust temperature. e1 1 TL TL TH1 1 e 550 273 K 1 0.28 592.6 K TH1 TL e1 T1 TL T TH1 592.6 1 e K 550 273 K 1o 0.28 o 592.6 L e2 1 L T 912 K 639 C 640 C H1 TH2 TH2 1 e2 1 0.35 TL TL 592.6 K e2 1 TH2 912 K 639o C 640o TH2 1 e2 1 0.35 5/23/2017 APHY201 11