* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ketogenesis (Biosynthesis of ketone bodies)
Survey
Document related concepts
Microbial metabolism wikipedia , lookup
Mitochondrion wikipedia , lookup
Metalloprotein wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Specialized pro-resolving mediators wikipedia , lookup
Biosynthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Citric acid cycle wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Transcript
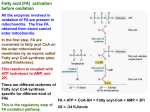
Ketogenesis (Biosynthesis of ketone bodies) • In humans, liver mitochondria have capacity to divert any excess acetyl-CoA formed in the liver during oxidation of fatty acids or oxidation of pyruvate that exceed capacity of citric acid cycle to undergo conversion to the ketone bodies. • ketone bodies : [acetoacetate, D-β-hydroxybutyrate& acetone (non metabolizable side product)] for export to other tissues, where they can reconvert to acetyl CoA & oxidized by citric acid cycle. 1 Ketone bodies are important sources of energy for the peripheral tissues because: 1. They are soluble in aqueous solution (don't need to be incorporated into lipoproteins or carried by albumin like lipid). 2. Produced in liver during periods when acetyl-CoA present exceed the oxidative capacity of the liver. How 3. They are used in proportion to their concentration in the blood by extrahepatic tissues (skeletal & cardiac muscle & renal cortex). 4. Brain, heart & muscle can use ketone bodies to meet their energy needs if the blood levels rise sufficiently (during prolonged periods of fasting). 2 Why ketone bodies synthesized by the liver: The production and export of ketone bodies from the liver to extrahepatic tissues allow continued oxidation of fatty acids in the liver when acetylCoA is not being oxidized in the citric acid cycle. 3 Synthesis of ketone bodies 1-Formation of acetoacetyl CoA can occur by one of 2 processes: a. Incomplete breakdown of fatty acid. b. Enzymatic condensation of two molecules of acetylCoA, which catalyzed by thiolase (the reversal of thiolase reaction of fatty acid oxidation). 2- The acetoacetyl-CoA, condenses with 3rd molecule of acetyl-CoA to form β -hydroxy- β -methylglutarylCoA (HMG-CoA) catalyzed by HMG-CoA synthase (the rate limiting step in the synthesis of ketone bodies & present in significant quantities only in the liver). 3- HMG-CoA is cleaved to free acetoacetate and acetylCoA catalyzed by HMG-CoA lyase. 4 4-The acetoacetate is reversibly reduced by D-β-hydroxy-butyrate dehydrogenase with NADH as hydrogen donor to β –hydroxybutyrate or it can be spontaneously decarboxylated to form acetone. Note: D-β-hydroxy-butyrate dehydrogenase [mitochondrial enzyme, specific for the D stereoisomer]. 5 Utilization of ketone bodies by peripheral tissues • Liver constantly produces low levels of ketone bodies, but their production becomes much more significant during starvation, when ketone bodies are needed to provide energy to the peripheral tissues. • Liver actively produces ketone bodies, but it can't utilize it as a fuel because [can not reconvert acetoacetate to acetyl CoA]. • In extrahepatic tissues, acetoacetate is activated to acetoacetyl-CoA by succinyl CoA - acetoacetate CoA transferase which transfer CoA portion from succinyl-CoA to acetoacetate to form acetoacetyl-CoA . • Extrahepatic tissues, including the brain, heart & muscle ,efficiently oxidize acetoacetate & 3-hydroxy butyrate. 6 Utilization of ketone bodies including the following steps: 1- 3-hydroxy butyrate is oxidized to acetoacetate by 3hydroxy butyrate dehydrogenase, producing NADH. 2- Acetoacetate receives a coenzyme A from succinyl CoA by the action of succinyl CoA - acetoacetate CoA transferase [succinyl CoA transferase] present in all tissues except the liver ? its absence allows the liver to produce ketone bodies but not utilize them, this ensures that extrahepatic tissues have access to ketone bodies as a fuel source during prolonged fasting & starvation. 3- Acetoacetyl CoA is actively removed by its conversion to two molecules of acetyl CoA by the action of thiolase. 7 Ketogenesis is regulated at three crucial steps: (1)Partition of acetyl-CoA between the pathway of ketogenesis and the citric acid cycle. • fall in concentration of oxaloacetate, within the mitochondria, impair the ability of the citric acid cycle to metabolize acetylCoA and divert fatty acid oxidation toward ketogenesis. • Such a fall occur because of an increase in the [NADH]/[NAD+] ratio caused by increased β-oxidation affecting the equilibrium between oxaloacetate and malate and decreasing the concentration of oxaloacetate. 8 (2) Free fatty acid mobilization from adipose tissue ;directly affects the level of ketogenesis . the factors regulating mobilization of free fatty acids from adipose tissue are important in controlling ketogenesis. 3) The activity of carnitine palmitoyl transferase- I in liver, which determines the proportion of the fatty acid flux that is oxidized rather than esterified; How 9 • carnitine palmitoyl transferase-I (CPT-I), regulate entry of fatty acids into the oxidative pathway (mitochondria). • CPT-I activity is low in the fed state, leading to decrease fatty acid oxidation, and , high in starvation, allowing fatty acid oxidation to increase • Malonyl-CoA, the initial intermediate in fatty acid biosynthesis, formed by acetyl-CoA carboxylase in the fed state, is a potent inhibitor of CPT-I. This will lead to decrease entry of fatty acids the mitochondria and all esterified to acylglycerol (decrease production of acetyl-coA). 10 • CPT-I activity is high in starvation, allowing fatty acid oxidation to increase. • as the concentration of free fatty acids increases with the onset of starvation, acetyl- CoA carboxylase is inhibited and malonyl-CoA decreases, CPT-I transport more of acyl-CoA to mitochondria to be oxidized (increase production of Acetyl-CoA). • These events are reinforced in starvation by decrease in the [insulin]/[glucagon] ratio. • Thus, β-oxidation is controlled by the CPT-I gateway into the mitochondria, and the balance of the free fatty acid uptake not oxidized is esterified. 11 12