* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download SeaWater properties
Indian Ocean wikipedia , lookup
Anoxic event wikipedia , lookup
Marine pollution wikipedia , lookup
Marine habitats wikipedia , lookup
Ocean acidification wikipedia , lookup
Arctic Ocean wikipedia , lookup
Global Energy and Water Cycle Experiment wikipedia , lookup
Hyperthermia wikipedia , lookup
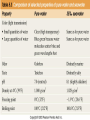
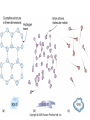
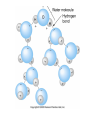
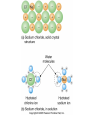
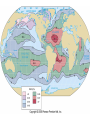
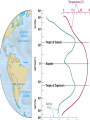
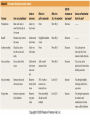
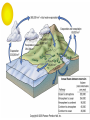
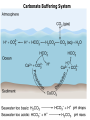
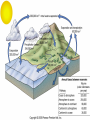
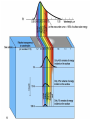
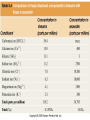
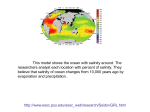
Mid Atlantic Ridge – new findings: • http://www.noc.soton.ac.uk/gg/classroom @sea/JC007/about.html • http://www.sciencedaily.com/releases/200 7/03/070301103112.htm http://www.noc.soton.ac.uk/gg/classroom@s ea/JC007/background.html Properties of Seawater WORLDS WATER SOURCES: Learning Objectives 1. Understand the nature of the water molecule and its unique properties (polarity, density and thermal properties) and how these are altered by the presence of salt in solution. Review of: Atoms Temperature Heat Thermodynamics Internal Energy and Entropy 2. Know the types of materials that are dissolved in sea water, their importance and how they vary with time. 3. Explain variations in salinity, temperature, and pressure within the sea and how they alter the chemical and physical properties of the ocean. States of Matter (e.g. water) States of Matter (e.g. water) Atomic Structure http://www.dayah.com/periodic/Other/Periodic%20Table.pdf Hydrogen bonds – cohesion - surface tension! The formation of ice in freshwater: Density of freshwater: Seawater density depends on temperature, salinity and pressure! Therefore, it increases with > salt content at const. temp; high density in cold, salty waters –why is this important? Why does ice float on water? Water is a powerful solvent: (“the universal solvent”) Sodium Chloride Rock SALT NaCl Na Cation Cl Ions Anion Cycling of dissolved components in seawater: Did oceans’ salinity increase over time? Major dissolved components in seawater: 35 g of salt in 1000 g of seawater The layer of rapidly changing salinity with depth; 3001000 meters; Same as pycnocline (density) and thermocline; Salinity map showing areas of high salinity (36 o/oo) in green, medium salinity in blue (35 o/oo), and low salinity (34 o/oo) in purple. Salinity is rather stable but areas in the North Atlantic, South Atlantic, South Pacific, Indian Ocean, Arabian Sea, Red Sea, and Mediterranean Sea tend to be a little high (green). Areas near Antarctica, the Arctic Ocean, Southeast Asia, and the West Coast of North and Central America tend to be a little low (purple). http://www.biosbcc.net/ocean/marinesci/02ocean/swcomposition.htm pH = potential/power of hydrogen Carbonate buffering system keeps the pH of seawater constant = 8.1 Carbonate Buffering System Waters Thermal Properties What is temperature? What is temperature? using Kinetic temperature definition What is temperature? It is a direct measure of the average kinetic energy of atoms and molecules that make up substance. Temp. changes when heat energy is added to or removed from a substance. It is measured in (Celsius, Kelvin, and Fahrenheit). HEAT (the energy of moving molecules = kinetic energy) 1) Represents the transfer of energy from high to low temperature. Therefore, heat has units of Energy (1 calorie, calor = heat; the amount of heat required to raise the temp. of 1 gram of water by 1 C°); 2) An object does not possess "heat"; the appropriate term for the microscopic energy in an object is internal energy. First Law of Thermodynamics Heat Capacity – the amount of heat required to raise the temp. of 1 g of any substance by 1 °C; – Water has one of the highest heat capacities known, which makes water excellent heat transfer material; and – therefore, allows ocean currents to moderate global climate! Atmospheric transport of surplus heat from low latitudes into heat deficient high latitudes areas: Learning Objectives: Ocean Sediments 1. Understand the origin and classification of marine sediments. 2. Explain the factors controlling origin and deposition of sediment on the continental shelf and in the deep ocean. Questions: 1. Why don't the oceans have more sediment in them? Where does it all go? Earth is 4.6 billion years old and the oceans should have more sediment in them. 2. Salt composition of the oceans has not changed for the last 1.5 billion years. Explain why? Learning Objectives: Plate tectonics and Ocean floor Understand the processes that are continuously changing Earth’s surface as lithospheric plates move relative to one another. Identify the role of oceanic ridges, transform faults and deep-sea trenches in defining the edges of lithospheric plates. Understand the importance of asthenospheric thermal convection in plate tectonics and the resulting compression or tensional forces at the plate boundaries. Explain the distribution of magnetic anomaly stripes, seismicity, and volcanism in terms of the concept of global plate tectonics. Spreading rates of ocean basins. Difference between oceanic and continental crust.