* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Thermal Energy & Heat THERMAL ENERGY & MATTER
Dynamic insulation wikipedia , lookup
Space Shuttle thermal protection system wikipedia , lookup
Insulated glazing wikipedia , lookup
Intercooler wikipedia , lookup
Underfloor heating wikipedia , lookup
Thermoregulation wikipedia , lookup
Heat exchanger wikipedia , lookup
Solar water heating wikipedia , lookup
Thermal comfort wikipedia , lookup
Passive solar building design wikipedia , lookup
Heat equation wikipedia , lookup
Thermal conductivity wikipedia , lookup
Building insulation materials wikipedia , lookup
Cogeneration wikipedia , lookup
Copper in heat exchangers wikipedia , lookup
Solar air conditioning wikipedia , lookup
Hyperthermia wikipedia , lookup
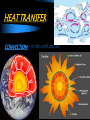
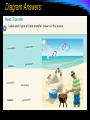
Thermal Energy & Heat THERMAL ENERGY & MATTER: Journal 1. 2. 3. 4. 5. 6. In which direction does heat flow spontaneously? Define TEMPERATURE How is THERMAL ENERGY transferred? What are the factors that determine the THERMAL ENERGY of a material? Which type of material heats more, one with a high specific heat, or one with a low specific heat? Is WORK 100% efficient? How do you know? THERMAL ENERGY & MATTER Work and Heat- work is never 100% efficient. Some is always lost to heat. THERMAL ENERGY & MATTER Heat- the transfer of thermal energy from one object to another because of a temperature difference. In what direction does heat flow spontaneously? FROM HOT to COLD THERMAL ENERGY & MATTER Temperature = measure of how hot or cold something is compared to a reference point. Temperature is the average kinetic energy of the particles in an object. In the image below, where is average kinetic energy greater? Higher temperature THERMAL ENERGY & MATTER Thermal energy- total potential and kinetic energy in an object. It depends on mass, temperature, and phase of an object. If both objects are in the same phase & at the same temperature, which one has MORE thermal energy? Because there are MORE particles moving around. THERMAL ENERGY & MATTER Specific Heat – amount of heat needed to raise ONE gram of a material ONE degree Celsius. THERMAL ENERGY & MATTER The LOWER a material’s specific heat the MORE its temperature rises when energy is added. Which will heat faster (has the lower specific heat)? Water? Specific heat of water = 4.18 J/g°C Or Lead? YES! Specific heat of lead = 0.46J/g°C Heat Transfer: 3 methods of heat transfer: conduction, convection, and radiation. Crust forming on the surface of lava is an example of a change of state by transferring heat from the hot lava to the10 cooler air surrounding the lava. HEAT TRANSFER What type of HEAT TRANSFER is occurring in the pictures? Conduction, convection or radiation? CONDUCTION – The transfer of thermal energy with no transfer of matter. Must be Touching! HEAT TRANSFER What type of HEAT TRANSFER is occurring in the pictures? Conduction, convection or radiation? CONVECTION – The transfer of thermal energy when particles of a liquid or gas move from one place to another HEAT TRANSFER CONVECTION – in the earth and sun HEAT TRANSFER What type of HEAT TRANSFER is occurring in the pictures? Conduction, convection or radiation? RADIATION – The transfer of thermal energy by waves moving through space. ALL OBJECTS radiate energy! THERMAL ENERGY & MATTER: Journal Define Convection, Conduction and Radiation 2. Give an example of each. 3. Write a sentence describing how each is important to our everyday lives. 4. How do we use heat in our everyday lives? 1. Where does heat transfer on this beach? Label at least four examples of heat transfer on the picture. 16 Diagram Answers: 17 Methods of Heat Transfer Think-Pair- Share!! Discuss examples of each type of heat transfer with a partner for two minutes. 18 Methods of Heat Transfer Convection: Heat transferred by the motion of a substance Conduction: Heat transferred by touching 19 Radiation: Heat transferred through as waves through space