* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download No Slide Title
Ore genesis wikipedia , lookup
History of geology wikipedia , lookup
Great Lakes tectonic zone wikipedia , lookup
Age of the Earth wikipedia , lookup
Large igneous province wikipedia , lookup
Geology of Great Britain wikipedia , lookup
Sedimentary rock wikipedia , lookup
Algoman orogeny wikipedia , lookup
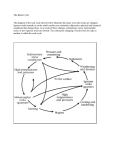
Chapter 2 Earth Materials— Minerals and Rocks Earth Materials – Minerals • Gemstones and other minerals, – such as gold, – have fascinated people for thousands of years – and have been supposed – to have mystical or curative powers • Minerals have many essential uses – in industrial societies • Minerals are the basic units – that make up most of Earth’s materials Earth Materials – Rocks • With only a few exceptions rocks – are solid aggregates of minerals • Rocks too find many uses – rocks crushed for aggregate in cement and for roadbeds – sawed and polished rocks for tombstones, monuments, mantle pieces and counter tops – Even the soils we depend on • for most of our food • formed by alteration of rocks Rocks • Granite cliff of El Capitan, Yosemite, California – rises 900 m above the valley floor – highest unbroken cliff in the world Minerals • Geological definition of a mineral: – naturally occurring – crystalline solid • crystalline means that minerals • have an ordered internal arrangement of their atoms – minerals have a narrowly defined chemical composition – and characteristic physical properties such as • density • hardness • color... Minerals Minerals on display – at the California Academy of Sciences in San Francisco Earth Materials • Some materials formed by the Earth – are interesting and attractive – such as this metamorphic rock • from the shoreline of Lake Superior at Marquette, Michigan Matter and Its Composition • Matter – is anything that has mass and occupies space – exists as solids, liquids, and gases – consist of elements and atoms • Element – – – – is a chemical substance that cannot be chemically decomposed into simpler substances and is composed of tiny particles called atoms Atoms • Atoms are the smallest units of matter – that retain the characteristics of the element • Atoms have – a compact nucleus containing • protons – particles with a positive electrical charge • neutrons – electrically neutral particles – particles orbiting the nucleus • electrons – negatively charged particles Structure of an Atom • The dense nucleus of an atom – consisting of protons and neutrons – is surrounded by a cloud of orbiting electrons Atoms • Atomic number = the number of protons • Atomic mass number = number of protons + number of neutrons • The number of neutrons in an atom – may vary Isotopes • The different forms of an element’s atoms – with varying numbers of neutrons – are called isotopes. • Different isotopes of the same element – have different atomic mass numbers – behave the same chemically • Isotopes are important in radiometric dating Carbon Isotopes • Carbon atoms (with 6 protons) – – – – have 6 neutrons = Carbon 12 (12C) have 7 neutrons = Carbon 13 (13C) or have 8 neutrons = Carbon 14 (14C) thereby making up three isotopes of carbon. Electrons and Shells • Electrons orbit the nucleus in one or more shells • The outermost shell participates – in chemical bonding – and contains up to 8 electrons • Noble gas configuration of 8 electrons • or 2 for helium – completes the outermost shell • Other atoms attain – a noble gas configuration – in the process of bonding Bonding and Compounds • Bonding – the process whereby atoms join to other atoms • Compound – a substance resulting from the bonding – of two or more elements • Oxygen gas (O2) is and element • Ice is a compound – made up of hydrogen and oxygen (H2O) • Most minerals are compounds Ionic Bonding • Ion – an atom that has gained or lost one or more electrons – and thus has a negative or positive charge • One way for atoms to attain the noble gas configuration – is by transferring electrons, producing ions • Ionic bonding – attraction between two ions of opposite charge Covalent Bonding • Another way for atoms – to attain the noble gas configuration – is by sharing electrons • Covalent bonding – results from sharing electrons shared electrons Minerals—The Building Blocks of Rocks • A mineral’s composition is shown by a chemical formula – a shorthand way of indicating how many atoms of different kinds it contains – Quartz consists of Quartz: SiO2 1 silicon atom for every Ratio: 1: 2 2 oxygen atoms – Orthoclase consists of KAlSi3O8 1 potassium, 1 aluminum, and 3 silicon for every 1: 1: 3: 8 8 oxygen atoms Native Elements • A few minerals consist of only one element. • They are not compounds. • They are known as native elements. • Examples: – gold – formula: Au – diamond – formula: C Crystalline Solids • By definition, minerals are crystalline solids – with atoms arranged in a specific 3D framework • If given enough room to grow freely, – – – – minerals form perfect crystals with planar surfaces, called crystal faces sharp corners straight edges Narrowly Defined Chemical Composition • Some minerals have very specific compositions – examples are halite (NaCl) or quartz (SiO2) • but others have a range of compositions – because one element can substitute for another – if the atoms of the two elements have • the same electrical charge • and are about the same size – example: olivine • (Mg,Fe)2SiO4 • iron and magnesium substitution in any proportion Mineral Properties • Mineral properties are controlled by – Chemical composition – Crystalline structure • Mineral properties are particularly useful – for mineral identification and include: • • • • color streak luster crystal form • • • • cleavage fracture hardness specific gravity How Many Minerals Are There? • • • • More than 3500 minerals are known Only about 2 dozen are particularly common Many others are important resources Mineral groups: – minerals with the same negatively charged ion or ion group – belong to the same mineral group • Most minerals in the crust – belong to the group called silicates Silicates • Silicates are minerals containing silica – Si and O • They make up perhaps 95% of Earth’s crust – and account for about 1/3 of all known minerals • The basic building block of silicates – is the silica tetrahedron • which consists of one silicon atom • surrounded by four oxygen atoms Types of Silicates • Silica tetrahedra can be – isolated units bonded to other elements – arranged in chains (single or double) – arranged in sheets – arranged in complex 3D networks Types of Silicates • Ferromagnesian silicates – contain iron (Fe), magnesium (Mg), or both • Nonferromagnesian silicates – do not contain iron or magnesium Ferromagnesian Silicates • Common ferromagnesian silicates include – olivine – Hornblende, an amphibolegroup mineral – augite, a pyroxenegroup mineral biotite mica Nonferromagnesian Silicates Quartz Potassium feldspar Plagioclase feldspar Muscovite Other Mineral Groups • Carbonates – minerals with carbonate ion (CO3)-2 – as in calcite (CaCO3), • found in limestone – and dolomite [CaMg(CO3)2], • found in dolostone • Other mineral groups are important, – but more as resources – than as constituents of rocks Rock-Forming Minerals • Most rocks are solid aggregates – of one or more minerals • Thousands of minerals occur in rocks, – but only a few are common – and called rock-forming minerals • Most rock-forming minerals are silicates, – but carbonates are also important • Accessory minerals are present in small amounts – and are ignored in classifying rocks Rock Cycle • The rock cycle is a pictorial representation – of events leading to – the origin, destruction, change – and reformation of rocks • Rocks belong to 3 major families – igneous – sedimentary – metamorphic • The rock cycle shows – how these rock families are interrelated – and can be derived from one another Rock Cycle Pyroclastic material Lava Igneous Rocks • All igneous rocks – cool and crystallize from magma, – solidify from lava, – or consolidate from pyroclastic materials • Magma is molten material – below the surface • Lava is molten material on the surface • Pyroclastic materials – are particles such as volcanic ash Igneous Part of the Rock Cycle Pyroclastic material Lava Categories of Igneous Rocks • Extrusive or volcanic rocks – formed at the surface – from lava or pyroclastic materials • Intrusive or plutonic rocks – formed from magma injected into the crust – or formed in place in the crust • Plutons are intrusive bodies – consisting of plutonic rock Plutons Igneous Rock Textures • Texture – is the size, shape and arrangement – of crystals, grains and other constituents of a rock • Igneous rocks have 4 textures – that relate to cooling rate of magma or lava 4 Cooling-Rate Textures • phaneritic, – with visible grains • cooled slowly • aphanitic, – with grains too small to see without magnification • cooled quickly • porphyritic, – with larger grains surrounded by a finer-grained groundmass • cooled slowly first, then more quickly • glassy, – with no grains • cooled too quickly for minerals to grow Igneous Rock Textures • Other textures reveal further details – of the formation of the rock • Vesicular texture, with holes (vesicles), – indicates the rock formed – as water vapor and other gases – became trapped during cooling of lava • Pyroclastic or fragmental texture, – containing fragments, – formed by consolidation of volcanic ash – or other pyroclastic material Igneous Rock Textures Rapid cooling Slow cooling 2-stage cooling Aphanitic texture Phaneritic texture Porphyritic texture Igneous Rock Textures Glassy texture Vesicular texture Pyroclastic texture cooling was too rapid for mineral growth gasses trapped in cooling lava particles fragmented during eruption Classifying Igneous Rocks • Texture and composition are the criteria – used to classify most igneous rocks • Composition categories are based on silica content – felsic (>65% silica) – intermediate (53-65% silica) – mafic (45-52% silica) • More felsic magmas have higher Na, K, Al • More mafic magmas have higher Ca, Fe, Mg Classifying Igneous Rocks Common Igneous Rocks Basalt Gabbro Andesite Diorite Common Igneous Rocks Rhyolite Granite Classifying Igneous Rocks with Special Textures Texture Composition Felsic Vesicular Pumice Glassy Obsidian Pyroclastic or Fragmental Mafic Volcanic breccia Tuff/welded tuff Igneous Rocks with Special Textures Tuff has pyroclastic texture. Pumice is glassy and extremely vesicular. Sedimentary Rocks • Sedimentary rocks form – by the lithification of sediment • In the rock cycle, sediment originates when: – mechanical and chemical weathering • breaks rocks down into smaller particles • and into solution – Transport removes sediment • from its source area • and carries it elsewhere – Running water, glaciers, wind and waves • transport sediment – Deposition involves settling of particles, • and chemical and biological extraction of minerals from solution Sedimentary Part of the Rock Cycle Lithification • Lithification means – turning loose sediment into rock • Lithification occurs by – burial • when additional sediment accumulates on top – compaction • reduction of the amount of pore space between particles • because of the weight of overlying sediment – cementation • precipitation of minerals within pores • that effectively binds sediment together – calcium carbonate (CaCO3) cement is common – silica (SiO2) cement is common – iron oxide (Fe2O3) cement is less common Categories of Sedimentary Rocks • Detrital sedimentary rocks – consist of solid particles – derived from preexisting rocks (detritus) • Chemical sedimentary rocks – consist of minerals derived from materials in solution and – extracted by either • inorganic chemical processes • or by the activities of organisms – subcategory biochemical sedimentary rocks, for which • the activities of organisms are important Detrital Sedimentary Rocks • are composed of fragments or particles – known as clasts = Clastic texture • These rocks are defined primarily by size of clasts • conglomerate – composed of gravel (>2mm) – with rounded clasts • sedimentary breccia – also composed of gravel (>2mm) – but clasts are angular • sandstone – composed of sand Detrital Sedimentary Rocks • Mudrocks consist of particles < 1/16 mm – siltstone • composed of silt-sized particles - 1/16-1/256 mm, • feel slightly gritty, • but not visible without magnification – mudstone • composed of a mixture of silt- and clay-sized particles – claystone • composed of clay-sized particles – <1/256 mm, feel smooth even to the teeth – shale • mudstone or claystone that • breaks along closely spaced parallel planes (fissile) Chemical Sedimentary Rocks • Recall that these rocks result – when inorganic chemical processes – or organisms extract minerals from solution • This can result in different textures – Crystalline texture • has an interlocking mosaic of mineral crystals • results from chemical precipitation – Clastic texture • has an accumulation of broken pieces of shells Chemical Sedimentary Rocks • Limestone – carbonate rock made of calcite precipitated chemically or by organisms • Dolostone – carbonate rock made of dolomite usually formed from limestone • Evaporites formed by – inorganic chemical precipitation during evaporation – Rock salt – evaporite made of halite – Rock gypsum – evaporite made of gypsum • Chert – compact, hard, fine grained silica, formed by chemical or biological precipitation (some consisting of microscopic shells of silica-secreting organisms) • Coal – made of partially altered, compressed remains of land plants accumulated in swamps Common Sedimentary Rocks Conglomerate Quartz sandstone Sedimentary breccia Shale Common Sedimentary Rocks Rock gypsum Fossiliferous limestone Rock salt Chert Coal Metamorphic Rocks • Metamorphic rocks – result from transformation of other rocks – in the solid state, without melting • Changes resulting from metamorphism – compositional • new minerals form – textural • minerals become reoriented • minerals recrystallize – or both Metamorphic Part of the Rock Cycle Agents of Metamorphism • Heat provides new conditions – where different minerals may be stable – and increases the rate of chemical reactions • Pressure – Lithostatic pressure provides new conditions • where different minerals may be stable • and forms smaller denser minerals – Differential pressure • exerts force more intensely from one direction • causing deformation • and development of foliation. • Fluid activity enhances metamorphism – by increasing the rate of chemical reactions – by transporting ions in solution Types of Metamorphism • Contact metamorphism – – – – heat chemical fluids from an igneous body alter rocks adjacent to the magma • Regional metamorphism – – – – – large, elongated area tremendous pressure elevated temperatures fluid activity occurs at convergent and divergent plate boundaries Metamorphic Textures • Foliated texture – platy and elongate minerals aligned parallel to one another – caused by differential pressure • Nonfoliated texture – – – – mosaic of roughly equidimensional minerals or platy and elongate minerals arranged in a helter-skelter fashion with random orientations Formation of Foliation • When rocks are subjected to differential pressure – the minerals typically rearrange or grow parallel to each other Formation of Foliation • Microscopic view – of a metamorphic rock – with foliation – showing the parallel arrangement of minerals Foliated Metamorphic Rocks • Slate – very fine-grained, breaks in flat pieces • Phyllite – fine-grained (coarser than slate but grains are still too small to see without magnification) – breaks in flat pieces • Schist – clearly visible platy and/or elongate minerals • Gneiss – alternating dark and light bands of minerals Nonfoliated Metamorphic Rocks • Marble – made of calcite or dolomite from limestone or dolostone • Quartzite – made of quartz from quartz sandstone • Greenstone – made of green mafic igneous rock • Hornfels – results from contact metamorphism • Anthracite – made of black lustrous carbon from coal Common Metamorphic Rocks Slate Gneiss Schist Marble Quartzite Plate Tectonics and the Rock Cycle • The atmosphere, hydrosphere and biosphere – act on earth materials – and cause weathering erosion and deposition • Earth’s internal heat – aids melting and metamorphism • Plate tectonics recycles Earth materials – heat and pressure at convergent plate boundaries • lead to metamorphism and igneous activity – resulting deformation makes mountains • that in turn weather and erode to form sediment Earth Materials and Historical Geology • Our record of Earth’s history – is preserved in rocks • Sedimentary rocks are especially useful – in preserving a historical record – and will be covered in more detail later in the term • Igneous rocks reveal much – about the history of plate activity • Metamorphic rocks provide information – about processes deep in the crust Summary • Elements consist of atoms, – which have a nucleus • of protons and neutrons – surrounded by orbiting electrons in shells • The number of protons = the atomic number • and the number of protons + neutrons = the atomic mass number • Bonding of atoms occurs – by transfer of electrons to make ions • ionic bonding – or by sharing electrons • covalent bonding Summary • Most minerals are compounds – of two or more elements bonded together • The most common minerals are silicates, • which contain silicon and oxygen, – but carbonate minerals, • with carbon and oxygen – are abundant in some rocks • Two broad groups of igneous rocks – are intrusive (or plutonic) – and extrusive (or volcanic) • Igneous rocks are classified primarily – by composition and texture Summary • Sedimentary rocks also have two broad groups – detrital, • which consist of solid particles of preexisting rocks, – and chemical/biochemical, • which consist of minerals derived • by inorganic or organic (involving organisms) processes • Compaction and cementation – transform sediment into sedimentary rock, – in a process called lithification Summary • Metamorphic rocks form – when composition and/or texture – of another rock changes – by heat, pressure and fluid activity • Metamorphism imparts a foliation – to many rocks, • which is a parallel alignment of minerals • Some metamorphic rocks have a mosaic – of equidimensional minerals – and are nonfoliated • Texture or composition – are the primary considerations – in classifying many metamorphic rocks