* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Nitrogen and Phosphorus Cycles
Photosynthesis wikipedia , lookup
Crop rotation wikipedia , lookup
Conservation agriculture wikipedia , lookup
Constructed wetland wikipedia , lookup
Perovskia atriplicifolia wikipedia , lookup
Triclocarban wikipedia , lookup
Lake ecosystem wikipedia , lookup
Renewable resource wikipedia , lookup
Sustainable agriculture wikipedia , lookup
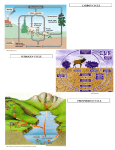
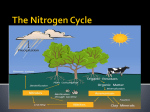
Nutrient Cycles Nitrogen and Phosphorus WHY DO WE NEED NITROGEN?? – Nitrogen is needed to make up DNA and protein! • In animals, proteins are vital for muscle function. • In plants, nitrogen is important for growth. NITROGEN Nitrogen “stores” Largest store = atmosphere (N2) Also stored in.... • Oceans • Organic matter (in soil) NITROGEN 78% of the planet’s Nitrogen is N2 (atmosphere) there are 3 main ways that nitrogen is made available to plants/ animals.... #1 – Nitrogen Fixation – This is a process that changes N2 into forms that plants can use! (nitrate) (ammonium) Happens in atmosphere... LIGHTNING! Happens in soil, and in water bodies Lightning provides the energy for nitrogen to react with oxygen in the atmosphere! Nitrogen-fixing bacteria in the soil can convert (“fix”) N2 to ammonium. Nitrogen-fixing cyanobacteria in water can also do this! In the water In the soil Rhizobium Usually live on roots of legumes and other plants. Video Nitrogen fixing Inbacteria the soil – are an example of SYMBIOSIS!! These bacteria grow on the root nodules of legumes like peas. The plants provide sugars, while bacteria provide nitrogen ions. #2 – Nitrification and #3- Uptake In the soil, nitrifying bacteria convert: NH4+ NO2- NO3Nitrate can be taken up by plant roots! (Uptake) So, plants that don’t live with nitrogen fixing bacteria, can also get nitrogen from the soil. The Nitrogen Cycle How does N2 get back to the atmosphere? Denitrification = changes NO3- back to N2 This occurs in aquatic and land ecosystems by denitrifying bacteria • Excess nitrogen dissolves in water, enters the waterways, and washes into lakes and oceans. The nitrogen compounds eventually become trapped in sedimentary rocks and will not be released again until the rocks undergo hundreds of years of weathering. The Nitrogen Cycle • Human activities can also affect the nitrogen cycle. – Due to human activities, the amount of nitrogen in the ecosystem has doubled in the last 50 years. – Burning fossil fuels and treating sewage releases nitrogen oxide (NO) and nitrogen dioxide (NO2). • Burning also releases nitrogen compounds that increase acid precipitation in the form of nitric acid (HNO3). Acid rain damaged these trees • Agricultural practices often use large amounts of nitrogen-containing fertilizers. – Excess nitrogen is washed away, or leaches, into the waterways. • This promotes huge growth in aquatic algae called algae blooms. • Algae blooms use up all CO2 and O2 and block sunlight, killing many aquatic organisms. • Algae blooms can also produce neurotoxins that poison animals. A nice summary... The Phosphorus Cycle • Phosphorus is essential for life processes in plants and animals. – Phosphorus is a part of the molecule that carries energy in living cells = ATP (cellular respiration)!! The Phosphorus Cycle • How do plants and animals use phosphorus? Plants Developing healthy seeds, root growth, and stem strength! Corn with a Phosphorus deficiency Animals (humans) Developing healthy bones (works with Ca to build bone tissue) Where do we find P? • It is not stored as a gas in the atmosphere like C and N.... • P is stored in phosphate rock and sediments on the ocean floors (Phosphates: PO43-, HPO42-, and H2PO4-) How it gets from rock soil Phosphorus is released from rock into the soil by a process called “weathering” CHEMICAL PHYSICAL -Acid rain - Chemical in lichens -Wind, rain, freezing Weathering = rock breaks down into smaller pieces. These pieces make their way into the soil. The Land Cycle • Plants take up phosphate through their roots • Animals eat the plants (get phosphate) • Decomposers return it to the soil The Aquatic Cycle • Phosphate gets into the water by erosion, leaching, run-off • Most settles at the bottom (turns into sediment) • Some phosphate is taken up by aquatic plants Geological Uplift • Mountains and hills are created when rock gets “uplifted” • The earth’s crust folds (very slowly) and deeply buried rock layers rise up Mt. Everest is made of limestone that must have originally formed on ancient sea floor. It contains fossils of marine creatures. This is a rock in Scotland. Below the yellow line was once horizontal rock. It has been uplifted over time. It has now started to erode due to weathering. Human Activity & the P Cycle • We affect the P cycle by: – Mining phosphate rock (for fertilizers and detergents) – Making fertilizers and detergents (industrial waste) – Applying fertilizer to land – Fishing (remove aquatic organisms – small effect) A Nice Summary How Changes in Nutrient Cycles Affect Biodiversity • Any significant changes to any of these nutrients (C, H, O, N, or P) can greatly affect biodiversity. – Carbon cycle changes contribute to climate change and global warming. • Slight temperature fluctuations and changes in water levels can drastically change ecosystems. • Changes influence other organism in the food webs. How Changes in Nutrient Cycles Affect Biodiversity – Increased levels of nitrogen can allow certain plant species to outcompete other species, decreasing resources for every species in the food webs. – Decreased levels of phosphorus can inhibit the growth of algae that are very important producers in many food chains.