* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Listeris, Legionella, and small gram
Cryptosporidiosis wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Surround optical-fiber immunoassay wikipedia , lookup
Trichinosis wikipedia , lookup
Hepatitis B wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Oesophagostomum wikipedia , lookup
Onchocerciasis wikipedia , lookup
Typhoid fever wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Marburg virus disease wikipedia , lookup
Chagas disease wikipedia , lookup
Rocky Mountain spotted fever wikipedia , lookup
Leishmaniasis wikipedia , lookup
Meningococcal disease wikipedia , lookup
Visceral leishmaniasis wikipedia , lookup
Schistosomiasis wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Brucellosis wikipedia , lookup
Leptospirosis wikipedia , lookup
Coccidioidomycosis wikipedia , lookup
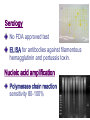
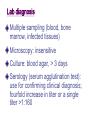
Miscellaneous Small Gram-Negative Bacilli Yu Chun-Keung DVM, PhD Department of Microbiology and Immunology College of Medicine National Cheng Kung University Chapter 36 Bordetella Chapter 37 Brucella Francisella Chapter 35 Haemophilus Most of the G(-) bacilli are commonly found in the environment or as normal members of the human microbial flora. The isolation of Bordetella, Brucella and Francisella is always associated with disease. Bordetella 博德氏菌屬 Extremely small (0.2 x 1 μm ), G(-), coccobacilli Fastidious growth requirement (charcoal, starch, blood or albumin to absorb toxic substance in medium, eg. agar) Bordetella B. pertussis: whooping/pertussis (severe cough) B. parapertussis: mild form of pertussis B. bronchiseptica: respiratory disease of animals (pigs and dogs) The three species are closely related, differing only in the expression of virulence genes Pathogenesis • Exposure (aerosol) • Bacterial attachment to ciliated epithelial cells of the respiratory tract • Proliferation • Production of toxins • Localized tissue damage and systemic toxicity Colonization of tracheal epithelial cells by Bordetella pertussis 2004 Kenneth Todar University of Wisconsin-Madison Department of Bacteriology Virulence Factors Adhesins (黏附因子) Toxins Bacterial adhesins Filamentous hemagglutinin (絲狀紅血球凝集素): contain RGD motif: (1) sulfated glycoprotein integrins on ciliated respiratory cells; (2) CR3 on macrophages. Pertactin (P69 protein) : contain RGD motif Pertussis toxin: A classic A-B toxin. Toxic subunit (S1) and binding subunit (S2 to S5); S2 binds ciliated respiratory cells, S3 binds phagocytic cells Fimbria : mediate binding in vitro; function unknown Toxins S1 subunit of pertussis toxin: increase respiratory secretion Adenylate cyclase/hemolysin toxin: increase respiratory secretion, inhibit leukocyte function Dermonecrotic toxin: vasoconstriction and tissue destruction Tracheal cytotoxin: ciliostasis, extrusion of ciliated cells, impair regeneration of damaged cells (disrupt clearance mechanism, lead to cough), IL1 production (lead to fever) LPS: unknown (stimulate cytokine release) A tracheal organ culture 72 h after infection with B. pertussis. Large arrow: Bordetella Small arrow: cilia Extruded epithelial cell with attached bacteria Denuded epithelium of non-ciliated cells Clinical disease Infect ciliated epithelial cells of the airways, produce disease locally, no invasion. Catarrhal phase (卡他期): sneezing, serous rhinorrhea, malaise, low-grade fever, 1-2wk, infectious. Paroxysmal phase (突發期): repetitive coughs and inspiratory whoop, vomiting and exhaustion, 40-50 paroxysms daily, bronchopneumonia, 2-4wk. Recovery phase (恢復期): lasts for above 3 wks. Epidemiology Pertussis has been considered a pediatric disease (< 1 year) Incidence (morbidity and mortality) has been reduced considerably after the introduction of vaccine in 1949. Still endemic worldwide. Immunity is not lifelong. Infection is seen in nonimmune infants, young children and adults with waning immunity. Incidence vs. Prevalence Epidemic vs. Endemic Lab diagnosis - culture Extremely sensitive to drying, cannot survive outside the host or traditional transport medium. Inoculate to freshly prepared medium at bedside. Charcoal-horse blood agar or Regan-Lowe charcoal medium with horse blood, glycerol, peptones. Nasopharyngeal aspirate (use synthetic fiber swabs not cotton swabs, fatty acid are toxic to Bp). 35°C, humidified, 7 days, sensitivity 50%. Diagnosis - microscopy Aspirated specimen Direct and indirect fluorescent antibody tests for antigen detection Sensitivity 50% 檢體玻片風乾熱固定 螢光抗體染色 Serology No FDA approved test ELISA for antibodies against filamentous hemagglutinin and pertussis toxin. Nucleic acid amplification Polymerase chain reaction sensitivity 80-100% Treatment Erythromycin during catarrhal phase (在卡 他期無法做正確診斷,失去治療先機). Primarily supportive, antibiotics do not ameliorate clinical course. Recovery depends on regeneration of ciliated epithelial cells. Prophylaxis for unimmunized infants (Pertussis is highly contagious, family members will become carrier). Vaccination DTP vaccine (inactivated whole cell of Bp, toxoid of tetanus and diphtheria), 80-85% effective. Vaccine has not been widely accepted because of vaccine-related complications. Acellular vaccine with inactivated pertusis toxin and filamentous hemagglutinin, pertactin or fimbriae. Chapter 37 Brucella 布魯氏桿菌屬 Francisella 法蘭西氏菌屬 α-Proteobacteria Brucella Rickettsia Ehrlichia γ-Proteobacteria Francisella Legionella Pasteruella Pseudomonas Brucella (布魯氏桿菌) Small, 0.5 1.5 M, G(-) Fastidious, grow slowly on culture (>1 week) Zoonotic pathogens and potential agent of bioterrorists Brucella B. melitensis : goats and sheep B. suis : swine B. abortus : cattle B. canis : dogs, fox Sources of Brucella infection. G.G. Alton & J.R.L. Forsyth Brucellosis (Bang’s disease, undulant fever, Malta fever) B. melitensis : severe disease in humans B. suis : severe and chronic B. abortus : mild disease B. canis : mild disease Pathogenesis Obligate intracellular parasites of animals and humans Infect monocytes/macrophages, replicate in phagolysome under the control of virulence genes in virB operon Spread to spleen, liver, lymph node bone marrow, kidneys (granuloma) Granuloma Clinical disease Acute stage: incubation period >2 months, fever rises in afternoon, fall during night with drenching sweat (undulant fever), weakness, malaise, chill, weight loss, nonproductive cough, aches, pain. Chronic stage: involve many tissues for many years, granulomas and abcesses. 70% GI symptoms, 20-60% bone lesions, 25% respiratory tract symptoms. Epidemiology Worldwide distribution Animal reservoirs; natural hosts develop mild or asymptomatic disease. Animal tissues (breast, uterus, epididymis, placenta) contain erythritol (紅鮮醇)which is required for the growth of the organism. Epidemiology In animals: sterility, abortion, and asymptomatic carriage. Milk, urine and birth products contain high number of bacteria. Human infections: Direct contact (a lab exposure) Ingestion: consume unpasteurized milk, milk products or cheese Inhalation Lab diagnosis Multiple sampling (blood, bone marrow, infected tissues) Microscopy: insensitive Culture: blood agar, > 3 days Serology (serum agglutination test): use for confirming clinical diagnosis; fourfold increase in titer or a single titer >1:160 T/P/C Tetracycline + doxycycline, bacteriostatic drugs, relapse is common; use doxycycline + rifampin for > 6 weeks. Control of disease in livestock Identification (serologic testing) Elimination of infected herds Vaccination Avoidance of unpasturized dairy products Observance of safety procedures in clinical lab Francisella tularensis (法蘭西氏土倫桿菌) Highly contagious: extremely hazardous for physician and lab workers. G(-), very small size (0.2 0.7 m), able to penetrate through skin and mucous membrane + aerosols Fastidious growth requirement (iron and cysteine) Distribute 20° North Pathogenesis Animal reservoirs: wild mammals (rabbits, hares, voles), domestic animals, bird, fish, bloodsucking arthropods (ticks) Human infections result from arthropod biting (10 organisms), direct contact (10), inhalation (50), or ingestion (108). Intracellular parasite, can survive for prolonged periods in macrophages; inhibit phagosomelysosome fusion. Pathogenic strains possess antiphagocytic capsule. Tularemia (土倫病) (Rabbit fever / Tick fever) Fever, chills, malaise, fatigue Clinical symptoms and prognosis determined by route of infection Ulcer Cutaneous tularemia infection microbes.historique.net Ulceroglandular form: skin, most common Oculoglandular form: eyes, painful conjunctivitis. Typhoidal form: blood, sepsis with multi-organ involvement Pneumonic form: respiratory tract Gastrointestinal form: oral Clinical sign Clinical symptom Syndrome (eg. AIDS) Chapter 35 Haemophilus Pasteurellaceae (巴斯德桿菌科) Haemophilus (嗜血桿菌屬) Actinobacillus (放線桿菌屬) Pasteurella (巴斯德桿菌屬) Haemophilus “Blood-loving”, small G(-) bacilli, obligate parasite of mucus membrane. growth require factor x (hemin) and factor v (NAD); heated-blood (chocolate) agar for isolation. Haemophilus H. influenzae (流行性感冒嗜血桿菌) H. parainfluenzae H. ducreyi (杜克氏嗜血桿菌) H. aegyptius H. influenzae biogroup aegyptius Classification Polysaccharide capsular antigens: Type a – f Biotypes I to VIII (biochemical properties): indole production, urease activity, ornithine decarboxylase activity Two biogroups (clinical presentation): Hi biogroup aegypticus causes Brazilian purpuric fever Pathogenesis Nonencapsulated Hi / H. parainfluenzae Colonize URT in all people 10% of the flora of saliva: H. parainfluenzae Opportunistic pathogens: cause acute and chronic otitis and sinusitis, exacerbation of chronic bronchitis Pathogenesis - Encapsulated Hi type b Uncommon in the URT Common cause of disease in children Adhesins colonization of oropharynx release cell wall components damage and impair ciliary function Produce IgA1 proteases, facilitate colonization Major virulence factor: antiphagocytic polysaccharide capsule – (PRP: polyribitol phosphate) Anti-PRP antibody is protective (enhance phagocytosis and complement-mediated bacteriocidal activity) Phagocytic engulfment of H. influenzae bacterium opsonized by antibodies specific for the capsule and somatic (cell wall) antigen. 2004 Kenneth Todar University of WisconsinMadison Department of Bacteriology Absence of anti-PRP antibody (complement depletion, splenectomy ) leads to invasion, bacteremia and dissemination Clinical diseases Meningitis: Hi type b was the most common cause of pediatric meningitis. Cannot be differentiated from other causes of bacterial meningitis (S. pneumoniae, N. meningitidis, E. coli). Epiglottitis: swelling of the supraglottic tissue, rapidly progress to complete obstruction of the airways, life-threatening emergency. Cellulitis: reddish-blue patches on the cheeks or periorbital area. Arthritis: the most common form of arthritis (single large joint) in children <2 years old. Age-specific incidence of bacterial meningitis caused by Haemophilus influenzae, Neisseria meningitidis and Streptococcus pneumoniae prior to 1985 2004 Kenneth Todar University of Wisconsin-Madison Department of Bacteriology Epidemiology Before the introduction of vaccine, Hib was responsible for >95% invasive diseases, epiglottitis, orbital cellulitis, meningitis in children 5 m to 5 y (<3 m protected by maternal antibody). Hi type b conjugated vaccine was introduced in 1987 which greatly reduced the incidence of disease (>90%). Now infections occur in nonimmune children or adults with waning immunity. Hi type c and f and nonencapsulated strains become more common. The decline of Hib meningitis associated with the introduction of new vaccines Transmission Person-to-person transmission Increased disease frequency in households where there is a primary case or an asymptomatic carrier. Primary risk factor for invasive disease = absence of anti-PRP antibody. Close contacts should be given chemoprophylaxis. Diagnosis Samples: Cerebrospinal fluid and blood (>107 bacteria/ml) Microscopy: both sensitive & specific; G(-) bacilli in CSF in >80% cases before antibiotics treatment Culture: chocolate agar Satellite phenomenon: grow around colonies of Staph. aureus on unheated blood agar. Particle agglutination: detect PRP antigen, rapid and sensitive (1 ng/ml) Anti-PRP Ab-coated latex particles + specimen, if PRP present, “+” agglutination Treatment Prompt antimicrobial therapy, otherwise mortality 100% Serious infections: cephalosporins Less severe infections: ampicillin Antibiotic chemoprophylaxis (rifampin) for high risk group Hi type b conjugate vaccines Hib polysaccharide vaccine: not effective for < 18 months (CHO: no/low immunogenicity) Hib conjugate vaccine: purified capsular PRP + different carrier proteins: -Neisseria meningitidis outer membrane protein -Diphtheria toxoid Three doses of vaccine (the same type) before age of 6 months followed by booster doses. Haemophilus ducreyi (杜克氏嗜血桿菌) Cause chancroid (soft chancre,軟性下疳), a sexually transmitted disease. Indurated ulcer on genitalia with regional lymphadenopathy. Differential: syphilis, herpes simplex, lymphogranuloma venereum (Chlamydia trachomatis) H. aegyptius Purulent conjunctivitis H. influenzae biogroup aegyptius Brazilian purpuric fever