* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Antibiotics Part 1 - mededcoventry.com
Biosynthesis wikipedia , lookup
Signal transduction wikipedia , lookup
Genetic engineering wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Transformation (genetics) wikipedia , lookup
Point mutation wikipedia , lookup
Deoxyribozyme wikipedia , lookup
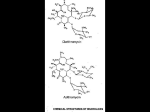
Antibiotics Part 1 Dr P Gayo Munthali Consultant Microbiologist UHCW Honorary Associate Clinical Professor University of Warwick Objectives • By the end of this lecture you should be able to: 1) Explain the mode of action of beta lactams, aminoglycosides, fluoroquinolones, macrolides, tetracyclines and glycopeptides 2) Mention the major side effects of the antibiotic groups in (1) 3) Appreciate different types of resistance and in simple terms, explain the mechanisms of resistance to beta lactams 4) Explain some limitations in the use of antibiotics in (1) 5) Understand the general spectrum of activity of antibiotics in (1) Antibiotics, Point of Action Folic acid Metabolism Trimethoprim, Sulphonamides Cell wall Synthesis Beta-lactams, Glycopeptides Daptomycin Fosfomycin Cell membrane Polymixin, bacitracin,colistin 50S 30S DNA Replication Quinolones Protein Synthesis DNA Dependent RNA 30S Tetracyclines,Aminoglycosides 50S Chloramphenicol, Clindamycin, Pol. Rifampicin Erythromycin, Linezolid,Streptogramin Important mechanisms of resistance to antibiotics Mechanism Typical example Antibiotics affected Inactivation ß-Lactamases ß-Lactams Aminoglycoside modifying enzymes Aminoglycosides Changes in Changes in ß-Lactams/ target binding PBPs/Peptide Terminal Glycopeptides Ribosomal methylation Macrolides Efflux and permeability changes DNA gyrase mutation Fluoroquinolones Efflux pumps Pump specific Porin protein loss Most except polymyxins and aminoglycoside ß-Lactams Β-Lactam Ring Thiazolidine Ring Penicillins and Cephalosporins s R-CONH Penicillins 1940- N o R-CONH s COOH R N o COOH Cephalosporins 1948- Carbapenems and Others CHз Carbapenems 1976- HO S R HO N o o COOH N o Clavulanic acid 1976 COOH Mobactams R Monobactam 1981- R-CONH N o R Mechanisms of Action • Inhibit bacterial enzymes involved in cell wall synthesis – Penicillin binding proteins (PBPs) essential for peptidoglycan synthesis • Trigger membrane associated autolytic enzymes that destroy cell wall • Inhibit bacterial endopeptidase and glycosidase enzymes which are involved in cell wall growth • Time dependent activity Beta Lactams Against Bacterial Cell Wall Cell wall Osmotic Pressure Cell Membrane Antibiotic against cell wall Osmotic Pressure Cell membrane Rapture Spectrum of Activity • • • • Very wide Gram positive and negative bacteria Anaerobes Spectrum of activity depends on the agent and/or its group • Aztreonam only active against gram negatives Pharmacokinetics • Absorption – PO forms have variable absorption – Food can delay rate and extent of absorption • Distribution – Widely to tissues & fluids – CSF penetration: IV – limited unless inflamed meninges IV 3rd & 4th generation cephalosporins, meropenem, & Aztreonam – penetrate well • Metabolism & Excretion – Primarily renal elimination – Some have a proportion of drug eliminated via the liver – ALL -lactams have short elimination half-lives Adverse Effects Penicillin hypersensitivity – 0.4% to 10 % – Mild: rash – Severe: anaphylaxis & death • There is cross-reactivity among all Penicillins • Penicillins and cephalosporins ~5-15% • Penicillins and carbapenems~1% (may be higher) – Desensitization is possible for mild hypersensitivity • Aztreonam does not display cross-reactivity with Penicillins and can be used in penicillin-allergic patients Resistance to ß-Lactams •Penicillin-Binding Protein (PBP) mediated Resistance •ß-Lactamase •Efflux pumps/loss of porins Penicillin-Binding Protein (PBP) mediated Resistance • • • • PBP over expression Acquisition of Foreign PBPs genes Mutation by recombination with foreign DNA Point mutation PBP over expression • Rare – The more PBPs are expressed, the more an organism becomes resistant • S.aureus increased resistance to methicillin by over expression of PBP4 • E.faecium strains that over express PBP5 have increased resistance to penicillin. AAC 39:2415-2422, AAC 38:1980-1983, AAC 45: 1480-1486 Acquisition of Foreign PBPs • Represented best by Methicillin Resistant S.aureus (MRSA) • S.aureus acquires foreign PBP2a encoded by mecA gene • PBP2a has low affinity for all ß-lactams • PBP2a can perform all the combined functions of all the S. aureus PBPs • Almost all MRSAs express ß-Lactamase Clin. Microbiol. Rev.10:781-791, J.Infect.Dis.162:705-710 Result • All PBPs in S.aureus become redundant –MRSA is resistant to all ß-lactams Mutation by Recombination with Foreign DNA • Streptococcus pneumoniae and Neisseria are capable of picking up foreign DNA and integrating it with their own DNA – Form mosaic gene • Pneumococcus picks up resistant genes from alpha haemolytic streps – Reduced affinity to beta lactams • Seen as penicillin resistant Pneumococci 9 8 7 6 MICs 5 Isolate Series1 Series2 BSAC Series3 MIC for meningitis 4 3 2 1 im e ro x ef u C Pi pe ra c illi n e ia x ef tr C ta x ef o C JAC 1992,30(3);279-288 on im e m M er op e ne ne ip e Im ni c illi n m 0 Pe MIC 90 Against 100 Isolates/BSAC MIC90 Resistance Breakpoints Beta Lactam Activity Against 100 Penicillin Resistant Pneumococci from Spain Efflux pumps/Loss of Porins • Important type of resistance in Pseudomonas – A combination of ß-Lactamase production and porin loss can lead to complex resistance pattern • Can lead to carbapenem resistance without carbapenemase production Porins and Pumps Porins Overexpressed Efflux pumps Adapted from Journal of Bacteriology, April 2006, p. 2297-2299, Vol. 188, No. 7 Resistance due to ß-Lactamases • Mode of action – Classification ß-Lactamase ß -pleated sheet-5 ά-helices AAC 39:2593-2601 Bound ß-lactam by ß-Lactamase ß-Lactamases-action R-CONH s C N o s R-CONH HOH Enzyme CH3 COOH Enzyme-Ser-OH o CH3 C O Ser N H CH3 CH3 COOH Annu.Rev.Microbiol.45:37-67 Beta Lactam Classification • You do not need to know the classification or detailed information on ß-Lactamases • However you need to appreciate the following concepts; – Simple betalactamases – Extended spectrum betalactamases (ESBL) – Betalactamases against the Carbapenems Simple ß-Lactamases • Many Based on genes called TEM-1 and SHV-1 found on mobile DNA elements – TEM-1 and SHV-1 are simple penicillinases in Enterobacteriaceae – Inactive against cephalosporins – Confer resistance to Penicillins such as Benzylpenicillin and amoxicillin – On mobile elements and therefore transmissible • Staphylococci also produce simple beta lactamases not based on TEM-1 and SHV-1 – Flucloxacillin designed to resist betalactamases in Staphylococcus aureus AAC 33:1131-1136 Extended Spectrum ß-lactamases • Based on TEM-1 and SHV-1 • Amino acid mutations in active site progressively increase their activity against cephalosporins – When they hydrolyze extended-spectrum cephalosporins • They are then called ESBLs – Also attack a monobactam Aztreonam -On mobile elements thus transmissible – Carry other resistance genes, Gentamicin, Ciprofloxacin ESBLs • Hydrolyze extended-spectrum cephalosporins with an oxyimino side chain • These include; – Cefotaxime – Ceftazidime – Ceftriaxone • Loose term • Among the beta-lactam, only the Carbapenems are stable against ESBLs – Imipenem, Meropenem, Ertapenem and Doripenem are in clinical use Characteristics of ESBLs • May appear sensitive to some cephalosporins and combinations of piperacillin and tazobactam as well as amoxicillin and clavulanic acid – However, use of these ß-lactam agents will lead to microbiological and clinical failure – Only carbapenems among the ß-lactams can be used successfully AmpC ß-Lactamases • Produced by almost all gram-negative bacteria • Chrosomally encoded versions important in Citrobacter freundii, Enterobacter aerogenes, Enterobacter cloacae, Morganella morganii, Pseudomonas aeruginosa and Serratia marcescens (not found in Salmonella and Klebsiella) • AmpC ß-Lactamase genes have been found on transferable plasmids Class C ß-Lactamases • All ß- lactams induce AmpC ß-lactamase production – Only carbapenems are resistant to AmpC ßlactamases • If there is loss of porins as well, this will lead to carbapenem resistance – Other ß- lactams will be hydrolysed Metallo-ß-Lactamases • Require Zinc or other heavy metal for activity • Hydrolyse all ß-Lactams including carbapenems • Most will be associated with resistance to many antibiotic classes • Currently New Delhi Metallo-ß-Lactamase (NDM-1) is a new flavour in the UK – Associated with India – Resistant to almost all antibiotics in use in the UK Aminoglycosides •Highly positively charged compounds, concentration dependent activity •Inhibit bacterial protein synthesis by irreversibly binding to 30S ribosomal unit •Naturally occurring: •Streptomycin •Neomycin •Kanamycin •Tobramycin •Gentamicin •Semisynthetic derivatives: •Amikacin (from Kanamycin) •Netilmicin (from Sisomicin) 30S Ribosomal Unit Blockage by Aminoglycosides •Causes mRNA decoding errors •Block mRNA and transfer RNA translocation •Inhibit ribosome recycling Ribosome recycling follows the termination of protein synthesis Spectrum of Activity • Gram-Negative Aerobes – Enterobacteriaceae; E. coli, K. pneumoniae, Proteus sp. Citrobacter, Enterobacter sp. Morganella, Providencia, Serratia – Pseudomonas aeruginosa – Acinetobacter • Gram-Positive Aerobes (Usually in combination with ß-lactams) S. aureus and coagulase-negative staphylococci Viridans streptococci Enterococcus sp. (gentamicin) Mechanisms of Resistance • Ribosome changes – Prevents binding • Loss of cell permeability • Expulsion by efflux pumps • Enzyme inactivation by Aminoglycoside modifying enzymes – This is the most important mechanism Pharmacokinetics • All have similar pharmacologic properties • Gastrointestinal absorption: unpredictable but always negligible • Distribution – Hydrophilic: widely distributes into body fluids but very poorly into; • CSF • Vitreous fluid of the eye • Biliary tract • Prostate • Tracheobronchial secretions • Adipose tissue • Elimination – 85-95% eliminated unchanged via kidney – t1/2 dependent on renal function – In normal renal function t1/2 is 2-3 hours Adverse Effects • Nephrotoxicity – Direct proximal tubular damage - reversible if caught early – Risk factors: High troughs, prolonged duration of therapy, underlying renal dysfunction, concomitant nephrotoxins • Ototoxicity – 8th cranial nerve damage – irreversible vestibular and auditory toxicity • Vestibular: dizziness, vertigo, ataxia • Auditory: tinnitus, decreased hearing – Risk factors: as for nephrotoxicity • Neuromuscular paralysis – Can occur after rapid IV infusion especially with; • Myasthenia gravis • Concurrent use of succinylcholine during anaesthesia Macrolides • Erythromycin is the prototype antibiotic for this group • Bacteriostatic- usually • Inhibit bacterial RNA-dependent protein synthesis • Bind reversibly to the 23S ribosomal RNA of the 50S ribosomal subunits – Block translocation reaction of the polypeptide chain elongation Macrolides Lactone Ring 14 14 Erythromycin 15 Azithromycin Telithromycin 14 Clarithromycin Mechanisms of Resistance • Altered target sites – Methylation of ribosomes preventing antibiotic binding • Resistance to macrolides , lincosamides (Clindamycin) and streptogramin B • Can be induced by macrolides • Efflux pumps – Resistance to macrolides only • Cross-resistance occurs between all macrolides Spectrum of Activity • Gram-Positive Aerobes: – Activity: Clarithromycin>Erythromycin>Azithromycin • MSSA • S. pneumoniae • Beta haemolytic streptococci and viridans streptococci • Gram-Negative Aerobes: – Activity: Azithromycin>Clarithromycin>Erythromycin • H. influenzae, M. catarrhalis, Neisseria sp. • NO activity against any Enterobacteriaceae • Anaerobes: upper airway anaerobes • Atypical Bacteria • Other Bacteria: Mycobacterium avium complex Pharmacokinetics 1 • Erythromycin ( Oral: absorption 15% - 45%) • Short t1/2 (1.4 hr) • Acid labile • Absorption (Oral) – Erythromycin: variable absorption of 15% - 45% – Clarithromycin: 55% – Azithromycin: 38% • Half Life (T1/2) – Erythromycin 1.4 Hours – Clarithromycin (250mg and 500mg 12hrly) 3-4 & 5-7 hours respectively – Azithromycin 68hours – Improved tolerability • Excellent tissue and intracellular concentrations – • • Tissue levels can be 10-100 times higher than those in serum Poor penetration into brain and CSF Cross the placenta and excreted in breast milk Pharmacokinetics 2 • Metabolism & Elimination – Clarithromycin partially eliminated by the kidney – ALL hepatic elimination Adverse Effects • Gastrointestinal (up to 33 %) (especially Erythromycin) • • • • Nausea Vomiting Diarrhoea Dyspepsia • Thrombophlebitis: IV Erythromycin & Azithromycin • QTc prolongation, ventricular arrhythmias • Other: ototoxicity with high dose erythromycin in renal impairment Fluoroquinolones Quinolone pharmacore Fluoroquinolones • Synthetic antibiotics • Concentration-dependent bactericidal activity • Broad spectrum of activity • Excellent pharmacokinetics • bioavailability, tissue penetration, prolonged half-lives • In common use • Ciprofloxacin • Levofloxacin • Moxifloxacin Mechanism of Action • Inhibit bacterial topoisomerases which is used by bacteria to; • Relax supercoiled DNA before replication • DNA recombination • DNA repair • DNA gyrase – Primary target for gramnegatives • Topoisomerase IV – Primary target for gram-positives Resistance • Altered target sites due to point mutations. • The more mutations, the higher the resistance to Fluoroquinolones • Most important and most common • Altered cell wall permeability • Efflux pumps • Cross-resistance occurs between fluoroquinolones Spectrum of Activity • Gram-positive (MSSA Streptococcus pneumoniae ) – Moxifloxacin is most active • Gram-Negative (Enterobacteriaceae H. influenzae, M. catarrhalis, Neisseria sp. Pseudomonas aeruginosa) – Ciprofloxacin is most active • Atypical bacteria: all have excellent activity Pharmacokinetics • Absorption • Good bioavailability • Oral bioavailability 60-95% • Divalent and trivalent cations (Zinc, Iron, Calcium, Aluminum, Magnesium) and antacids reduce GI absorption • Distribution • Extensive tissue distribution but poor CSF penetration • Metabolism and Elimination • Combination of renal and hepatic routes Adverse Effects • Cardiac • Prolongation QTc interval • Assumed to be class effect • Articular Damage • Cartilage damage • Induced in animals with large doses Tetracyclines •Hydronaphthacene nucleus containing four fused rings •Tetracycline •Short acting •Doxycycline •Long acting Mechanism of Action • Inhibit protein synthesis • Bind reversibly to bacterial 30S ribosomal subunits • Prevents polypeptide synthesis • Bacteriostatic Resistance • Efflux • Alteration of ribosomal target site • Production of drug modifying enzymes Spectrum of Activity • All have similar activities • Gram positives aerobic cocci and rods – Staphylococci – Streptococci • Gram negative aerobic bacteria • Atypical organisms – – – – Mycoplasmas Chlamydiae Rickettsiae Protozoa Pharmacokinetics • Incompletely absorbed from GI, improved by fasting • Metabolised by the liver and concentrated in bile (3-5X higher than serum levels) • Excretion primarily in the urine except doxycycline ( 60% biliary tract into faeces,40% in urine) • Tissue penetration is excellent but poor CSF penetration – Incorporate into foetal and children bone and teeth Avoid in pregnancy and children Adverse Effects • Oesophageal ulceration • Photosensitivity reaction Glycopeptides • Vancomycin • Teicoplanin Vancomycin Mechanism of Action • Inhibit peptidoglycan synthesis in the bacterial cell wall • Complex with D-alanyl-D-alanine portion of the cell wall precursor Resistance • Modification of D-alanyl-D-alanine binding site of peptidoglycan • D-alanyl-D-alanine terminal then ends in D-alanylD-lactate • Leads to lower glycopeptide affinity • Complex reactions to achieve this Spectrum of Activity • Gram positive bacteria only including MRSA Pharmacokinetics • Absorption • oral is negligible • IV required therapy for systemic infections • Distribution – Distributes widely into body tissues and fluids, including adipose tissue – Variable penetration into CSF, even with inflamed meninges • Elimination – Primarily eliminated unchanged by the kidney Adverse Effects • Red-Man Syndrome – Erythema multiforme-like reaction with intense pruritus, tachycardia, hypotension, rash involving face, neck, upper trunk, back and upper arms • Related to infusion rate • Resolves spontaneously after discontinuation • Lengthen infusion (over 2 - 3 hr) • Hematological – Neutropaenia – Eosinophilia