* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Nutraceuticals- Emerging Field of Metabolic Engineering of Lactic
Genetically modified food wikipedia , lookup
Gene prediction wikipedia , lookup
Biotechnology wikipedia , lookup
Designer baby wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Gene expression profiling wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Biochemistry wikipedia , lookup
Gene regulatory network wikipedia , lookup
History of biotechnology wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Cofactor engineering wikipedia , lookup
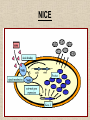
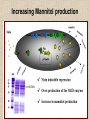
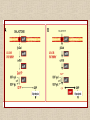
NUTRACEUTICALS: An emerging field for metabolic engineering of Lactic Acid Bacteria Nutraceuticals • The term ‘Nutraceuticals’, launched by Stephen De-Felici in the 1980s • A food or part of a food that may provide medicinal or health benefits, including the prevention and treatment of disease. Metabolic Engineering Metabolic engineering is the practice of optimizing genetic and regulatory processes within cells to increase the cells' production of a certain substance Controlled over expression of desired genes Inactivation of undesired genes Examples of metabolic engineering of LAB • Increased production of diacetyl from glucose and lactose • Efficient production of L-alanine from sugar • Production of non-metabolisable sugars • Galactose and/or lactose removal from dairy products • Oligosaccharide production • Vitamin production Lactic acid bacteria as cell-factories • Lactic acid bacteria (LAB) are industrially important microbes, used in a large variety of food fermentations • The NICE system for controlled heterologous and homologous gene expression in Lactic acid bacteria has been employed in many of the metabolic engineering strategies (Boels et al. 2001; Sybesma et al. 2002) Why Lactic acid bacteria? • The bacterium is food grade • Plasmid selection mechanisms are available that are food grade and self cloning • No endotoxins or inclusion bodies are formed and • Sophisticated genetic tools enable easy genetic handling • Simple, non-aerated fermentation makes direct scale-up from 1-L scale to 1000-L scale possible • Nisin controlled gene expression can be effectively used NICE Increased Vitamins Production • Folate – Involved in biosynthesis of nucleotides – Daily recommended intake for an adult is 200 µg – Known to prevent neural-tube defect in infants – Protect against some forms of cancer • Main sources are vegetables and dairy products • Milk is good source, fermented dairy products like yoghurt are also important • Streptococcus thermophilus and Lactococcus lactis execute de novo biosynthesis of folates to secrete surplus folate • Therefore can be used to make starter with increased folate levels • In experimental yoghurt up to 150 µg/L folate has been reported (Smid etal. 2001) Part of Folate gene cluster L. lactis cloned behind strong promoter • The genes involved in folate biosynthesis have been analysed completely. • By genetic eng. several of these genes have been over expressed in L.lactisNZ9000 using the NICE system • Individual gene can be over expressed or in combination • Folate normally synthesis as polyglutamyl-folate derivatives intracellularly • Absorbed in human guts as monoglutamyl folate derivatives • γ -glutamyl hydrolase cDNA introduced in L. lactis • Resulted in an inversion of folate spatial distribution (Sybesma et al. 2002) High production of folate by over expression of whole fol gene cluster Folate production in engineered Lb. gasseri Folate level in the organs of animals depleted in folate and supplemented with LAB folate Riboflavin (B2) • Riboflavin-deficiency can lead to:– Liver(Ross & Klein 1990) and skin-disorders (Lakshimi 1998) – Disturbed metabolism of the red blood cells (Hassan & Thurnham 1977) – Reduced performance during physical exercise (Belko et al. 1983; Bates 1987) • In Bacillus subtilis first reaction in riboflavin biosynthesis has been demonstrated to be rate limiting (Humbelin et al. 1999) • The gene coding for this enzyme, ribA, has been brought to overexpression in L. lactis using the NICE-system • This resulted in a 3-fold overproduction of riboflavin Production of non-metabolisable sugars • Mannitol and sorbitol (polyols) and trehalose could replace sucrose, lactose, glucose or fructose in food products • In colon they are fermented by micro-organisms to short-chain fatty acids (mainly butyrate) which may prevent colon cancer • Trehalose is therapeutic against illnesses, such as the Creutzfeld-Jakob disease • Mannitol and sorbitol have stool-bulking properties and can be used as dietary fibers • They are active as bifidogenic prebiotic • Cholesterol lowering , immunomodulant • They display equivalent sweetness and taste (Dwivedi 1978) • Mannitol can also serve as anti-oxidant in biological cells (Shen et al. 1997) Activation of Sorbitol production • Heterofermentative lactic acid bacteria such as Leuconostoc mesenteroides are known to produce mannitol in the fermentation of fructose (Soetaert et al. 1995) • homofermentative lactic acid bacteria can also produce mannitol • In both Lactobacillus plantarum (Ferain et al. 1996) and Lactococus lactis (Neves et al. 2000), disruption of lactate dehydrogenase (LDH) resulted in production mannitol along with other metabolites • Overproduction of the mannitol-P dehydrogenase (MPDH) in a LDH-deficient L. lactis strain has resulted in strong increase in intracellular mannitol production • Similar results were obtained when MPDH was overproduced in a strain with decreased phosphofructokinase (PFK) activity • Production of mannitol by Lactococcus lactis can be increased if excretion of this polyol is facilitated, by introducing the mannitol-transporter present in Leuconostoc mesenteroides. Increasing Mannitol production Effect of pH on the production of mannitol and sorbitol by Lb. plantarum VL202 Tagatose production • A potential sucrose replacement. • Higher sweetening power than similar components such as mannitol, sorbitol and erythritol • Much lower caloric value (Zehner 1988) • Recently been launched on the food market as low calorie sugar, as prebiotic Calorific values of different sugars • • • • Glucose Mannitol Sorbitol Erythritol 4.0 cal/gm 1.5 cal/gm 2.5 cal/gm 0.2 cal/gm • Chosen strategy is to disrupt the lacC and/or lacD genes resulting in production of either tagatose-6-P or tagatose-1,6-diphosphate • Disruption of lacD was accomplished via a two step procedure – recombination process, involving integration of an erythromycin-resistance plasmid containing only the lacC and lacF genes via single crossing-over – removal of lacD (or reversion to the wild-type) in a second, spontaneous, recombination event Production of polysaccharides • Exopolysaccharides (EPS) – Some polysaccharides produced by lactic acid bacteria have prebiotic (Gibson & Roberfroid 1995) – Immunostimulatory (Hosono et al. 1997) – Antitumoral (Kitazawa et al. 1991) – Cholesterol-lowering activity (Nakajima et al. 1992a) • The specific eps genes are encoded on large plasmids • Conjugally transferred from one lactococcal strain to the next, thereby introducing the EPS-producing capacity in the recipient strain ( van Kranenburg et al. 1997) Polysaccharide gene cluster in various LAB Improving sugar conversion • In cow’s milk 4–4.5% (w/v) of lactose present • In liquid fermented dairy products, such as yoghurt or buttermilk, usually less than half is fermented to lactic acid • These products are unsuitable for lactose intolerant persons • The lactose is converted to galactose and later to galactitol • For most lactic acid bacteria, galactose is a poor substrate • The efficiency lactose utilization by L.lactis can be increased by metabolic engineering • Secondly lactose metabolism in L. lactis can be modified in such a way that the glucose moiety will end up in the product, while galactose will be fully used for growth, in this way providing a natural sweetening process for dairy products Galactose of Lactose being fully utilized and Glucose ends up in the product • Free galactose is accumulated intracellularly as a result of the absence of galactokinase activity in these strains • Streptococcus thermophilus, gene for galactokinase is completely intact, but that one or more point mutations have taken place leading to a ‘silent’ phenotype (Vaughan et al. 2001). • Sometimes these mutations may revert back spontaneously • To enhance the galactose utilization these mutations can be reverted deliberately - Galactosides and their hydrolytic enzymes Removal of raffinose • Soy- and pulse-derived food products contain high levels of α-galactosides such as stachyose and raffinose • These are not metabolized in human gut due to lack of - galactosidase • These undigested - galactosides accumulate in the lower gut and induce gastric problems like flatulence • By applying metabolic engineering strategies, lactic acid bacteria can be constructed with high αgalactosidase activities • Starters for removal of α-galactosides during soy fermentation • Possible probiotics to deliver α-galactosidase activity in the gut for prevention of flatulence • In Lactobacillus plantarum gene (melA) code for α-galactosidase (Silvestroni et al. 2002) • For construction of starter and probiotic bacteria with high α-galactosidase activity, the melA is cloned in L. lactis in three different constructions resulting in – expression of the enzyme in the cytoplasm for maximum protection of enzyme activity – expression as a secreted enzyme for maximum exposure to the sugar substrate – expression on the surface but anchored to the surface of the cell Conclusion • Metabolic engineering has provided a powerful and effective tool for production of nutraceuticals • Metabolic engineering approach can also be applied for production of more benificial product. • With increasing knowledge of the genomic analysis metabolic engineering can further be explored for more nutraceutical production.