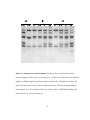
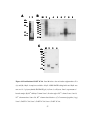
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CALPAIN: TRANSITIONING FROM THE USE OF THE
Two-hybrid screening wikipedia , lookup
Genetic code wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Biochemistry wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Catalytic triad wikipedia , lookup
CALPAIN: TRANSITIONING FROM THE USE OF THE PROTEASE CORE TO THE FULL-LENGTH ENZYME FOR THE DEVELOPMENT OF SPECIFIC SUBSTRATES AND INHIBITORS by JACQUELINE CHRISTINE KELLY A thesis submitted to the Department of Biochemistry In conformity with the requirements for the degree of Master of Science Queen’s University Kingston, Ontario, Canada (September, 2008) Copyright © Jacqueline Christine Kelly, 2008 Abstract Calpains are a family of calcium-dependent cysteine proteases involved in intracellular signaling. They participate in many normal cellular processes such as cell motility and apoptosis but when over-activated they contribute to diseases ranging from ischemic injury to neurodegenerative disorders. The major calpain isoforms µ- and m- are large heterodimeric enzymes that are subject to autoproteolysis and aggregation when activated by Ca2+. To avoid these complications the protease core (domains I and II) has been used to screen inhibitors and design substrates. Using the protease core of µ-calpain, I showed that the superior calpain substrate, PLFMER, is cut at the intended scissile bond between F and M. Alanine substitutions at each position optimized the sequence to PLFAAR, which has a 2.3-fold higher turnover rate. The set of substrates derived from this study provided a tool for profiling the activity of calpain isoforms. One disadvantage of the protease core is that it is less active than the whole enzyme. This was even more apparent with the protease cores of the tissue-specific calpains 3, 8, 9 and 15 such that it prevented their use in substrate and inhibitor screening. The recently solved crystal structure of calcium-bound full-length m-calpain has revealed additional sites for the interaction of substrates and inhibitors in the unprimed side of the catalytic cleft provided by domain III. To sample these sites, it is necessary to prevent the full-length calpain from aggregating and precipitating upon calcium binding. I have developed a method here that uses portions of calpastatin (CAST), the natural ii endogenous inhibitor and stabilizer of calpain, to keep the enzyme soluble. By artificially connecting those portions of calpastatin that bind to calpain domains IV and VI, it is possible to stabilize the enzyme without blocking its active site. Of the three constructs made, 1C-2A, 2C-3A, and 3C-4A, the 3C-4A peptide was shown to completely inhibit aggregation of m-calpain at a 1:1 molar ratio, as monitored by turbidity. This mechanism of stabilization will permit the use of full-length calpains for the development of specific substrates and inhibitors. iii Acknowledgements I would first and foremost like to acknowledge my research supervisor, Dr. Peter L. Davies from whom I have gained a great deal. Not only through his guidance in my research but also through his enthusiasm and love for the lab, have I appreciated the opportunity to be a part of his team. He has an incredible dedication to scientific research as well as the professional and personal development of the students and employees around him. It has been a great honour to have spent my graduate studies under his guidance and my future successes in research will be in a large part attributed to his mentorship. In my personal life, I would like to thank my loving husband, Shane Kelly, who has expressed his unwavering support and encouragement for my personal and academic decisions during the past 6 years of our marriage. I would also like to acknowledge my parents, Ron and Shirley Charles, as well as my brothers, Scott and Brian, for their love and support throughout my life. My immense appreciation goes out to the members, past and present, of the Davies lab that have made the last 2 years of my life both enjoyable and memorable: Rachel Hanna, Ravikiran Ravulapalli, Dr. Andy Scotter, Dr. Laurie Graham, Adam Middleton, Chris Garnham, Nelson Lin, Sally Yu, Kyra Nabeta, Jin Qian, Dr. Yee-Foong Mok, Jordan Chou and Kristin Low. I would like to extend special acknowledgement to: • The technical expertise and patience from Sherry Gauthier that helped facilitate the transition to my graduate studies. • The early guidance of Dr. Dominic Cuerrier in my research project and additional assistance even after pursuing his career. iv • The patience of Dr. Rob Campbell in teaching me crystallography and editing my work. Lastly, I would like to acknowledge Dr. Darrell Goll, the ‘father of calpain’, who passed away mid July of this year. I had the privilege of meeting Dr. Goll at a calpain conference and know that his enthusiasm and dedication to scientific research will live on through his written and spoken words. v Table of Contents Abstract............................................................................................................................................ii Acknowledgements.........................................................................................................................iv Table of Contents............................................................................................................................vi List of Figures.................................................................................................................................ix List of Tables ................................................................................................................................... x List of Abbreviations ......................................................................................................................xi Chapter 1 General Introduction ....................................................................................................... 1 1.1 Calcium Signaling.................................................................................................................. 1 1.2 Calpains ................................................................................................................................. 2 1.3 Calpain Family in Disease ..................................................................................................... 2 1.4 Calpain Structure ................................................................................................................... 5 1.5 The Mechanism of Activation of Calpain by Calcium .......................................................... 6 1.6 Tissue-Specific Calpains...................................................................................................... 10 1.7 Active-site Specificity and Substrate/Inhibitor Design........................................................ 12 1.8 Active-Site Nomenclature.................................................................................................... 13 1.9 Autolysis and Aggregation .................................................................................................. 14 1.10 Calpastatin ......................................................................................................................... 15 Chapter 2 Relative Activity of Calpains 3, 8, 9 and 15 and Inhibitor Profiling of Calpain 8........ 18 2.1 Preface ................................................................................................................................. 18 2.2 Abstract................................................................................................................................ 18 2.3 Introduction.......................................................................................................................... 19 2.4 Materials and Methods......................................................................................................... 20 2.4.1 Materials ....................................................................................................................... 21 2.4.2 Mutagenesis .................................................................................................................. 21 2.4.3 Relative Activity Measurements on Protease Cores ..................................................... 21 2.4.4 Inhibitor Profiling ......................................................................................................... 22 2.5 Results and Discussion ........................................................................................................ 22 2.5.1 Mini-calpains 3, 8 and 9 are Less Active than µI-II ..................................................... 23 2.5.2 Mutation of Mini-calpain 8 Increases its Activity ........................................................ 25 2.5.3 Mini-calpain 8 has Preferences for Leu, Ile and Val at the S2 Subsite......................... 25 vi Chapter 3 Profiling of calpain activity with a series of FRET-based Substrates ........................... 33 3.1 Preface ................................................................................................................................. 33 3.2 Abstract................................................................................................................................ 34 3.3 Introduction.......................................................................................................................... 34 3.4 Materials and Methods......................................................................................................... 36 3.4.1 Materials ....................................................................................................................... 36 3.4.2 Determination of the Cleavage Site within the PLFAER Substrate.............................. 37 3.4.3 Effect of Individual Residue Substitutions on Cleavage Kinetics using µI-II, µ- and mcalpain.................................................................................................................................... 37 3.5 Results and Discussion ........................................................................................................ 39 3.5.1 Determination of the Substrate Cleavage Site .............................................................. 39 3.5.2 Efficacy of Ala and Met at the P1΄ Position ................................................................. 42 3.5.3 Effect of Individual Residue Substitutions on Cleavage Kinetics ................................ 42 Chapter 4 The Stabilization of Calcium-bound Calpain by Calpastatin Peptides.......................... 48 4.1 Preface ................................................................................................................................. 48 4.2 Abstract................................................................................................................................ 49 4.3 Introduction.......................................................................................................................... 50 4.4 Materials and Methods......................................................................................................... 53 4.4.1 CAST C-A Peptide Cloning.......................................................................................... 53 4.4.2 Protein Expression and Purification.............................................................................. 54 4.4.3 CAST C-A Peptide Amino Acid Analysis.................................................................... 54 4.4.4 Monitoring Aggregation of mC105S by Turbidity ....................................................... 55 4.4.5 The Effect of CAST C-A Peptides on m-Calpain Activity........................................... 55 4.4.6 The Effect of CAST C-A Peptides on m-Calpain Autolysis......................................... 55 4.5 Results.................................................................................................................................. 56 4.5.1 Purification and Validation of the C-A Peptides........................................................... 56 4.5.2 CAST C-A Peptides Inhibit the Ca2+-Induced Insoluble Aggregation of mC105S ...... 58 4.5.3 CAST C-A Peptides Stabilize m-Calpain in the Presence of Ca2+ while Keeping the Active Site Clear .................................................................................................................... 62 4.6 Discussion............................................................................................................................ 65 Chapter 5 General Discussion........................................................................................................ 69 5.1 Is This the End of the Road for Mini-calpains? ................................................................... 69 vii 5.2 Full-Length Calpains, the Way of the Future ...................................................................... 71 5.3 Applications of Allosteric Inhibitor Design......................................................................... 74 5.4 Summary and Conclusions .................................................................................................. 76 viii List of Figures Figure 1.1. Mammalian calpain gene family. .................................................................................. 3 Figure 1.2. The catalytic triad of papain, Ca2+-free m-calpain and µI-II. ........................................ 7 Figure 1.3. Calcium-induced conformational change of m-calpain................................................. 9 Figure 1.4. Domain structure of CAST and its interaction with calpain........................................ 16 Figure 2.1. Inactive m-calpain (mC105S) hydrolysis activity assay of mini-calpains 1 (A), 8 (B), 3 (C), 9 (D) and 15 (E)......................................................................................................... 24 Figure 2.2. The effect of the stability of α7-helix on Trp106 (purple) in mI-II (A), µI-II (B), mcalpain (C) and mini-calpain 8 (D).. .................................................................................... 26 Figure 2.3. Autolysis assay of mini-calpain 8................................................................................ 27 Figure 2.4. Structure of the peptidomimetic epoxide library.. ....................................................... 29 Figure 2.5. SDS-PAGE of mC105S hydrolysis by mini-calpain 8. ............................................... 30 Figure 2.6. Profiling the S2 subsite of mini-calpain 8 with the natural amino acids. .................... 31 Figure 2.7. Profiling the S2 subsite of mini-calpain 8 with the non-natural amino acids.............. 32 Figure 3.1. NanoESI-MS spectrum of the cleavage products from the hydrolysis of (EDANS)EPLFMERK-(DABCYL) by µI-II....................................................................................... 40 Figure 3.2. Relative initial rates of the hydrolysis of the substituted FRET substrate series......... 45 Figure 4.1. The proposed mechanism of calpain stabilization using CAST.................................. 52 Figure 4.2. Purification of CAST 1C-2A....................................................................................... 57 Figure 4.3. Monitoring calpain aggregation by turbidity............................................................... 60 Figure 4.4. Monitoring the effect of the CAST C-A peptides on m-calpain activity and inhibition as measured with a FRET-based assay.. .............................................................................. 63 Figure 4.5. Autolysis of m-calpain in the presence of the CAST C-A peptides monitored by SDSPAGE. .................................................................................................................................. 64 ix List of Tables Table 3.1. Potential and observed FRET substrate hydrolysis products........................................ 41 Table 3.2. Preference for each amino acid at P1'-P3' positions of the hexapeptide substrate. ...... 43 Table 4.1. Validation of the CAST C-A amino acid analysis. ...................................................... 59 x List of Abbreviations AD, Alzheimer’s disease βME, 2-mercaptoethanol; CAST, calpastatin; COP, coatomer protein; DABCYL, 4-((4-(dimethylamino)phenyl)azo)benzoic acid; DMSO, dimethyl sulfoxide; DTT, dithiothreitol; E-64, trans-epoxysuccinyl-L-leucylamido (4-guanidino)butane; EDANS, 5-[(2-aminoethyl) amino]naphthalene-1-sulfonic acid; EDTA, ethylenediaminetetraacetic acid; FRET, fluorescent resonance energy transfer; HEPES, 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid; HPLC, high performance liquid chromatography; IFE, inner filter effect; LGMD2A, limb girdle muscular dystrophy type 2A; MES, 2-(N-morpholino)ethanesulfonic acid; MOPS, 3-(N-morpholino)propanesulfonic acid; MS, mass spectrometry; PEF, penta-EF-hand; SDS, sodium docecyl sulfate; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; Tris, tris(hydroxymethyl) aminomethane; xi Chapter 1 General Introduction 1.1 Calcium Signaling The role of calcium in cell signaling was serendipitously discovered over a century ago when Sidney Ringer was examining the contraction of isolated rat hearts [1]. The hearts would contract well when they were suspended in a saline medium in which he used tap water. However, he realized that when distilled water was substituted, the heartbeats became very weak and shortly thereafter ceased. He determined that it was the calcium in the tap water that was important in contraction of the heart muscle. Calcium is now recognized as the universal information carrier through the entire life cycle of a cell and its homeostasis is imperative to normal cell function, as both calcium depletion and overload leads to cell death via apoptosis or necrosis [2,3,4,5]. Another important feature of calcium is its self-regulation, as it is able to control its own influx, thereby effecting downstream events [6,7]. Calcium has also been shown to act as a primary messenger through activation of extracellular calcium-sensing receptors in the parathyroid and thyroid glands, kidney, osteoblasts, gastrointestinal mucosa, and recently in the nervous system [8]. The most important and best-characterized calcium-binding proteins are the EFhand family of proteins. These proteins bind calcium, inducing a conformational change which then relays the signal to other target proteins [9]. A classic example of this family 1 is calmodulin. Some of the calpains, the focus of this thesis, have penta-EF-hand (PEF) domains, in addition to the protease core with two non-EF-hand calcium-binding sites. These two sites act in concert with the PEF domains to cause a conformational change in calpain that leads to proteolytic cleavage of target substrates. 1.2 Calpains The name calpain stems from calcium ‘cal’, for its calcium binding characteristic, and papain ‘pain’, the classic, much-studied plant thiol protease [10]. Although calpain was named after papain, it actually has very low sequence identity to it and other cysteine proteases, and therefore has been distinguished as a CLAN CA, family C2, class of cysteine protease [11]. Calpain 1 (a.k.a. µ-calpain) was first discovered in rat brain in 1964 by Guroff [12], followed by the purification and partial characterization of calpain 2 (a.k.a. m-calpain) in 1976 [13]. Since that time, a total of 14 isoforms of calpain have been discovered in mammals (Fig. 1.1). The ubiquitous µ- and m-calpains are known to form heterodimers with the small subunit, calpain 4, and are named according to their calcium requirement (3-50 µM for µ-calpain and 400-800 µM for m-calpain) [14]. 1.3 Calpain Family in Disease Calpains are involved in many cellular processes including, but not limited to, cytoskeletal remodeling, cell motility [15], cell cycle progression [16], as well as apoptosis and necrosis [17]. Knockout studies have revealed the importance of calpain in 2 1, 2, 8, 9, 11,12,13,14 100 Small Subunit 3 5, 6 7 Atypical 10 15 Figure 1.1. Mammalian calpain gene family. The typical calpains 1, 2, 3, 8, 9, 11, 12, 13 and 14 are composed of an N-terminal variable region (red), protease core domains I (blue) and II (cyan), a C2-like domain III (green) and PEF domain IV (yellow). Calpains 1 and 2 form a heterodimer with the small subunit via the PEF domains IV and VI (orange). Calpains 5, 6, 7, 10 and 15 have domain deletions and additions, classifying them as atypical. Tissue-specific isoforms (3, 8, 9, 11 and 12) are shown in bold. The length of 100 amino acids is shown by the bar below DI of the typical calpains. 3 mice, as the absence of m-calpain [18] and the small subunit [19,20] are embryonically lethal. Surprisingly, µ-calpain knockout mice have normal long-term potentiation [21], but do lack proper platelet function [22]. The fact that µ-calpain knockouts are not embryonically lethal has lead to the hypothesis that µ- and m-calpains are functionally redundant and m-calpain may take the place of µ-calpain. However, more recently, calpain 1 has been localized to the intermembrane space of the mitochondria [23,24]. Thus, the functions of calpains 1 and 2 might be distinct because of compartmentation, with calpain 1 being principally involved in apoptosis. Calpain 3 knockout mice have been reported to have abnormal sarcomere formation [25] and calpain 10 knockout mice are viable but have not yet been characterized [26]. Calpains have been implicated in many pathological conditions, where calcium homeostasis is disrupted, leading to the mis-regulation of calpain. Chronic neurodegenerative disorders including Alzheimer’s [27,28], Parkinson’s [29] and Huntington’s [30] as well as ischemic brain, heart and kidney injuries [31] and traumatic brain and spinal cord injury are such conditions where calpain activity is up-regulated by a sustained increase in intracellular calcium. This results in both specific and non-specific cleavage of proteins involved in these processes. Although Alzheimer’s disease is far from completely understood, two hallmark events have been shown to occur, the formation of amyloid plaques and neurofibrillary tangles [32]. The most well-known calpain-specific event is the truncation of p35, the regulatory subunit and activator of the serine/threonine kinase Cdk5, by calpain to p25 4 [33]. p25 is more resistant to degradation than p35 and therefore accumulates in the brains of patients with AD and constitutively activates and mislocalizes Cdk5 [34]. The p25/Cdk5 complex has also been shown to display increased and altered tau phosphorylation, leading to reduced association of tau with microtubules and the formation of neurofibrillary tangles [34]. Since the implications of calpain in disease are extremely diverse and complex, it is difficult to determine whether its role is causal or coincidental. One disease where calpain is known to have a causal affect is limb girdle muscular dystrophy type 2A (LGMD2A), where mutations in the skeletal muscle-specific calpain 3 are known to cause this muscle wasting disease. Although our understanding of the roles of calpain in disease has come a long way in the past 40 years, a tremendous amount remains to be determined. 1.4 Calpain Structure The domain structure of the mammalian calpain gene family is shown in Fig. 1.1. The ubiquitous µ- and m-calpains have distinct large subunits, but share a common small subunit. The large subunits are composed of an N-terminal anchor helix that interacts with domain VI (DVI) of the small subunit in the absence of calcium (Fig.1.2A). This helix is amphipathic in µ-calpain and has been found to act as a mitochondrial targeting sequence [35]. This sequence is followed by the protease core, domains I and II, where the catalytic triad (Cys105, His262 and Asn286 in m-calpain) resides at the interface 5 [36]. DIII is a C2-like domain with an 8-stranded anti-parallel β-sandwich [37,38]. Following DIII is the PEF domain IV which has 5 EF-hand motifs, 4 of which bind calcium and the 5th forms the heterodimeric interface with the small subunit via pairing with the 5th EF-hand of DVI [37,39,40]. The small subunit shared by µ- and m-calpains is composed of two domains; a flexible, Gly-rich DV of unknown function and a PEF DVI, similar to DIV of µ- and m-calpains. With respect to protein structure, the calpain family can be classified as either typical or atypical (Fig. 1.1). The typical calpains (3, 8, 9, 11, 12, 13 and 14) have a domain structure similar to that of µ- and m-calpains, and are classified by the presence of a PEF domain IV. Although they had a PEF DIV, they do not necessarily heterodimerize with the small subunit. Calpain 3, for example, appears to form a homodimer of the large subunit, again through the 5th EF-hand of DIV [41]. On the other hand, the atypical calpains (5, 6, 7, 10 and 15) are classified based on domain additions or deletions to the typical subfamily, specifically, the deletion of the PEF domain IV (Fig. 1.1). 1.5 The Mechanism of Activation of Calpain by Calcium In papain, the active-site Cys and His are within 3.7 Å of each other, a distance that allows the His to deprotonate the Cys-SH, increasing its nucleophilicity (Fig. 1.2A). It was shown clearly from the crystallographic structure of m-calpain [37,42] that this equivalent inter-atomic distance in the calcium-free form is ~10.5 Å (Fig. 1.2B). This 6 A His159 Asp158 3.79 Å Cys25 B C Asn286 Asn296 His262 3.72 Å His272 10.64 Å Cys115 Ser105 Figure 1.2. The catalytic triad of papain, Ca2+-free m-calpain and µI-II. (A) The active site Cys25-S of papain is 3.79 Å away from His159-Nδ, demonstrating an aligned active site. (B) The unaligned active site of m-calpain. In the absence of calcium, Ser105-O is 10.64 Å away from His262-Nδ. (C) Calcium binding to calpain (µI-II shown here) causes a conformational change that aligns the active site by bringing Cys115-S within 3.72 Å of His272-Nδ. The protein backbone is coloured as shown in Fig. 1.1 [43]. 7 distance is much too far for acid-base catalysis to occur between these residues and provides an explanation for the inactivity of calpain in the absence of calcium [37]. Therefore, it was proposed that the major structural change upon calcium binding must be the alignment of the active site. Subsequently, the protease core of µ-calpain (µI-II) was shown to bind two calcium ions, one in each of DI and DII [44]. These bind cooperatively to peptide loops, which in turn cause conformational changes to the protease core that align Cys115 and His272 of the catalytic triad within 3.7 Å of each other, as seen in papain (Fig. 1.2C). Calpain was thought to bind one or more calcium ions to acidic loops on DIII [45] but this was not seen in the recent structure of calciumbound inactive m-calpain [46]. Up to four calcium ions bind to PEF domain VI in the small subunit [47,48], and by inference the same number were thought to bind to the homologous PEF domain DIV in the large subunit [42,44]. Conformational changes in DVI of the small subunit are very slight, with an 18° change in the angle between the first two α-helices of the first EF-hand (EF-1) exposing a hydrophobic surface [47,48]. Recent structural data from the co-crystallization of CAST and inactive calciumbound m-calpain have supported these prior claims (Fig. 1.3B) [46]. The predominant structural changes upon calcium binding do not occur within each domain, but in the relative positions of the domains to each other, resulting in a more compact protein. The changes in DI and DII are exactly the same as shown in the structure of µI-II, to align the active site [44]. In addition, the two PEF domains (IV and VI) are shifted up towards the active site, which displaces the N-terminal anchor helix [46]. As mentioned above, there 8 + Ca2+ - Ca2+ Figure 1.3. Calcium-induced conformational change of m-calpain. (A) In the absence of calcium, the N-terminal anchor helix interacts with DVI of the small subunit, and calpain is in an extended conformation. (B) Calpain binds two calcium ions in the protease core, one per domain, as well as four in each of the PEF domains DIV and DVI. Upon calcium binding, the N-terminal anchor helix is released from DVI, there is a rotation of DI relative to DII, and DIV and DVI are pushed closer to the protease core, resulting in an overall compaction of the enzyme. This is a dynamic process, as the addition of excess EDTA pushes this reaction back to the Ca2+-free form. The protein backbone is coloured as shown in Fig. 1.1. Calcium ions are illustrated as grey spheres [37,46]. 9 is exposure of hydrophobic pockets in DIV and DVI on binding calcium. These provide binding sites for the amphipathic helices in the conserved A and C sub-domains of CAST while the conserved B sub-domain binds to the newly formed catalytic cleft. These structural data have given scientists in the calpain field answers that have been long sought after, the conformational changes that occur in the entire enzyme upon calcium binding, as well as the mode of interaction and inhibition of calpain by its specific inhibitor, CAST. 1.6 Tissue-Specific Calpains The calpain family members can also be classified based on their expression, as either ubiquitous or tissue-specific. For example, the tissue-specific calpains 3, 8, 9, 11 and 12 are predominantly expressed in skeletal muscle, stomach, digestive tract, testes and hair follicles, respectively [49]. Calpain 3 (a.k.a. p94) has a similar domain structure to µ- and m-calpains (54 and 51% sequence identity respectively) [50], with the exception of two unique insertion sequences, IS1, in DII, and IS2, situated between DIII and DIV. It is extremely unstable when recombinantly expressed and undergoes rapid autolysis, which is avoided when it is expressed with the deletion of IS1 and IS2 [51]. This instability has also been shown during isolation of calpain 3 from skeletal muscle, while it remains stable in intact muscle. This property has been attributed to its interaction with connectin through IS2 [52]. As mentioned in section 1.3, mutations in the calpain 3 gene are known to cause limb girdle muscular dystrophy type 2A (LGMD2A) [53,54]. These mutations result in 10 calpain 3 deficiency that eventually leads to LGMD2A, although the exact mechanism by which this occurs is not known [55]. The stomach-specific calpain 8 (a.k.a. nCL-2) has been shown to be localized in pit cells of the stomach as well as goblet cells in the upper half of the villi in the duodenum [56]. A novel function for calpain 8 has been identified in membrane trafficking in mucus cells through its interaction with, and proteolysis of, β-COP of the COP1 coatomer complex [56]. The digestive tract-specific calpain 9 (a.k.a. nCL-4) is also located in goblet cells, although in the lower half of the villi [56]. Unlike most calpains, calpain 9 is down-regulated in human gastric cancer tissue [56,57]. Consistent with this result, mouse NIH3T3 fibroblast cells with antisense-induced calpain 9 deficiency, underwent cellular transformation and tumorigenesis [58]. The function and pathological roles of the tissue-specific calpains 11 and 12 remain elusive. Calpain 11 is expressed predominantly in the testis and has been postulated to be involved in the regulation of signal transduction events of cytoskeletal remodeling during meiosis, spermiogenesis and sperm function, based on its tissue location and temporal distribution [59]. Calpain 12 is highly expressed in the cortex of the hair follicle, but its function remains to be determined [60]. Due to the tissuespecificity of these calpains, they are promising targets for gene disruption and the elucidation of their function may be pharmacologically significant. 11 1.7 Active-site Specificity and Substrate/Inhibitor Design The development of calpain- and isoform-specific inhibitors is not only important for potential pharmacological treatments, but also for a greater understanding of the physiological roles of the calpain family members. The main problem associated with the development of calpain inhibitors is the cross-reactivity with other cysteine proteases, which share some common features in their active sites. The only known specific inhibitor of calpain is CAST, which interacts with heterodimeric calpains through the PEF domains IV and VI as well as the catalytic active-site cleft [46]. Although CAST has an extremely specific interaction with calpain, it is postulated only to inhibit the heterodimeric calpains, and then only after they have been activated by calcium. Even the individual inhibitory domains of CAST are too large at 10-15k Da to introduce into the cell as drugs and as intrinsically unstructured proteins they are extremely susceptible to proteolysis. An obvious target for inhibitor development is the active site. Small peptidomimetic molecules could potentially be developed that take advantage of small differences in preferences between each calpain family member. The crystal structure of µI-II has provided a means for the development of specific active-site inhibitors, as it is stable in the presence of calcium, thereby capturing an aligned catalytic triad [44]. Structures of µI-II in complex with inhibitors have suggested ways in which more specific calpain inhibitors can be produced [61]. The two archetypal cysteine protease inhibitors, E-64 and leupeptin, were shown to interact with only one side (unprimed) of 12 the active-site cleft of µI-II [43] and further studies revealed a way to design inhibitors that interact with both sides of the active site to provide increased specificity [62]. Calpains cleave extended regions in their target substrates but do not have absolute sequence specificity, unlike proteases such as trypsin or chymotrypsin. Nevertheless, calpains have preferences at each position of the active-site [63]. A librarybased approach has previously been used to determine these preferences at positions flanking both sides of the active site to produce optimal substrate and inhibitor sequences for µI-II [63,64,65]. This method can be extended to other calpain isoforms, in order to better understand their physiological function and develop isoform-specific therapeutic agents. 1.8 Active-Site Nomenclature The catalytic cleft of calpain contains an active cysteine, which undergoes covalent catalysis with its target substrates and, in some cases, its inhibitors. Flanking the cysteine are sites on both sides of the cleft, which can accommodate neighbouring residues of the substrate. For the purposes of clarity and consistency, Schechter and Berger proposed a nomenclature for the subsites of the catalytic cleft of proteases (S) as well as the positions of the sequences that interact with it (P) [66]. In the case of cysteine proteases, the region that is N-terminal to the active-site cysteine is designated as the unprimed side of the active site, with each subsite labelled S1-S3. The primed side of the active-site is the region that is C-terminal to the cysteine, and aptly named the S1’-S3’ 13 subsites. Similarly, the amino acid residues of target substrates have been labelled according to the subsite they interact with, P3-P3’. This nomenclature will be used throughout the thesis. 1.9 Autolysis and Aggregation As previously mentioned in section 1.5, calpain binds calcium in the protease core as well as domains IV and VI, thereby activating the enzyme through a structural change. Upon calcium binding, two hallmark events occur that make calpain difficult to work with: autolysis and aggregation. The events of autolytic cleavage of calpain are well documented and begin with the release of the N-terminal anchor helix, effectively lowering the calcium requirement of m-calpain from 400-800 µM to 50-150 µM [67]. The calcium requirement of µ-calpain is not lowered until the autoproteolytic release of DV of the small subunit, when it is decreased from 3-50 µM to 0.5-2.0 µM [68]. Further autolysis occurs in flexible loops between DII and DIII, which releases a calcium-dependent weakly active protease core, as well as between DIII and DIV. Calpain is unstable in the presence of calcium, even when it is inactivated by the mutation of the active-site cysteine to a serine. Previous structural studies have shown that calcium binding causes a conformational change in which hydrophobic patches are unveiled in domains IV and VI, providing possible sites for aggregation [69]. The mechanism by which aggregation occurs is very poorly understood and more insight 14 would be advantageous for crystallographic studies, as well as inhibitor and substrate specificity. This instability of calpain is known to be prevented by its endogenous inhibitor, CAST [70]. 1.10 Calpastatin Calpastatin (CAST) was first discovered in the mid 1970s when an unexpected lack of calpain activity was detected in porcine skeletal muscle [13]. This activity was not restored until calpain was isolated out of solution by precipitation [71]. The reason for this inactivity was the presence of a trypsin-labile, heat-resistant factor [71], later named CAST by Takashi Murachi in 1979 (see ref. [72]). It is a multidomain [73,74,75,76], unstructured protein [77] and the only known natural endogenous inhibitor that is specific for calpain [73]. CAST has at least 9 different isoforms due to the use of different promoters [78,79,80] as well as alternative splicing mechanisms [81,82]. They are each unstructured in aqueous solution and are typically composed of four homologous inhibitory domains (I-IV) and an N-terminal L (or XL in the case of one isoform) domain that has no inhibitory activity (Fig. 1.4A) [75]. It was later determined that in each domain, there are three conserved sub-domains: A, B and C [83,84]. Sub-domains A and C are known to form amphipathic α-helices upon the recognition of calpain in its calcium-bound form and interact with DIV of calpain and DVI of the small subunit, respectively [46,85,86]. This interaction is facilitated by the calcium-induced conformational change of calpain 15 A L 1 A B 2 C 190 191 A 3 B A C 323 324 B 4 A C 435 436 B C 572 573 713 B B A B C A A B B C C A A B B CC B A A C C Figure 1.4. Domain structure of CAST and its interaction with calpain. (A) Rat CAST is composed of an N-terminal L domain, followed by four inhibitory domains that each have A (turquoise), B (orange) and C (yellow) subdomains. Residue numbers marking the beginning of the A subdomain of each inhibitory domain are written below the bar diagram. (B) Graphical representation of CAST binding to four calpain molecules. The A (turquoise) and C (yellow) subdomains interact with DIV and DVI, respectively, and the B (orange) subdomain interacts with the calpain active site [87]. Calpain domains are coloured as in Fig. 1.1. 16 and resulting open conformation of EF-1 of both domains, exposing hydrophobic binding pockets for sub-domains A and C [46,69]. Domains I-IV were discovered to each bind calpain simultaneously, thereby providing inhibitory activity for up to four calpain molecules at once (Fig. 1.4B) [88,89]. There was some controversy over where the B sub-domain interacted [44,69,90], but with recent structural findings it was shown to be located in the active-site of calpain, where it exerts its inhibitory activity [46]. Since CAST interacts with the PEF domains in both the large and small subunits, it is expected to inhibit only those calpains that, first have a PEF domain IV, and secondly form a heterodimer with the small subunit. Currently, µ- and m-calpains are the only known typical isoforms that are inhibited by CAST. Since information is lacking on the other typical members of the calpain family as to whether or not they homo- or heterodimerize, it remains to be determined which of these would be expected to bind CAST. According to this classification, the atypical calpains (5, 6, 7, 10 and 15) would not interact with or be inhibited by CAST, as they lack a PEF domain IV (Fig. 1.1). The two largest obstacles in working with calpain have been its aggregation and autoproteolytic properties, as outlined in section 1.9. CAST seems to solve this problem by trapping calpain in a stable calcium-bound state, making it an excellent candidate to stabilize active, full-length calpain for in vitro experiments such as crystallography, inhibitor and substrate profiling and whole-cell screening. In order to do this, CAST would have to be re-routed out of the active site, to preserve calpain activity, which will be the focus of chapter 4 of this thesis. 17 Chapter 2 Relative Activity of Calpains 3, 8, 9 and 15 and Inhibitor Profiling of Calpain 8 2.1 Preface This chapter will be combined with additional information on the structure of minicalpain 8 solved by the Structural Genomics Consortium in Toronto, Ontario. Jacqueline Kelly was responsible for the mutagenesis of mini-calpain 8, activity assays of the mini-calpains as well as the profiling of the S2 position of mini-calpain 8. The experiments were performed under the supervision and guidance of Dr. Peter L. Davies with insight from Dr. Dominic Cuerrier. The protease cores were expressed and purified by Dr. Tara Davis at the Structural Genomics Consortium in Toronto, Ontario. 2.2 Abstract Calpains are calcium-dependent proteases that carry out limited proteolysis on many substrates in response to increases in intracellular calcium levels. Since various calpain isoforms are involved in pathological conditions such as Alzheimer`s disease, Type 2 diabetes, cancer, and LGMD2A, the development of specific small-molecule inhibitors is of pharmacological significance. The protease core of calpain 1 (µI-II) has been used to screen substrate and inhibitor libraries without the complications of autoproteolysis. Here we have explored the extension of this approach to a number of 18 tissue-specific calpains. The relative activities of several calpain cores were compared by looking at the time course for their digestion of inactivated m-calpain. One of the more active of these cores was that of stomach-specific calpain 8. Its inhibitor specificity was profiled with a synthetic peptidomimetic library of epoxide-based compounds that target the active site. The library probed the P2 position with both (R,R) and (S,S) stereochemistry of natural and non-natural amino acids. Calpain inhibition was analyzed by the extent of proteolysis of inactive m-calpain on an SDS-PAGE gel by the protease core of calpain 8. The stomach-specific calpain had similar inhibitor specificity at the P2 position as µI-II, particularly with the natural amino acids. However, the observed amino acid preferences for calpain 8 were weaker than for µ-calpain, which might reflect the overall lower activity of the calpain 8 core. 2.3 Introduction Calpains are a family of cysteine proteases that are activated by an increase in intracellular calcium levels. The human genome consists of 14 calpain isoforms that can be classified according to their expression, either ubiquitous (calpains 1, 2, 5, 7, 10, 13, 14 and 15) or tissue-specific (calpains 3, 8, 9, 11 and 12) [14,49,91]. Although the ubiquitous calpains 1 and 2 (aka. µ- and m-calpains, respectively) have been well characterized to date, much is to be learned of the remaining calpain isoforms. It is known that calpain 3 is expressed in skeletal muscle and is mutated in LGMD2A [53,54] and calpains 8 and 9 are predominantly localized to the stomach [92] and digestive tract [93], respectively. It has been proposed that, in the presence of calcium, calpain 8 cleaves 19 βCOP in pit cells and plays an important role in membrane trafficking of mucus cells [56,92]. Also, calpain 9 has been demonstrated to be down-regulated in gastric cancer [57,94] and has a role in the suppression of tumorigenesis [57,58,94]. The calpain gene family can also be broken down into typical, those with a similar domain structure to µ- and m-calpains, or atypical. Calpain 15 is classified as atypical with a distinctive domain structure and undetermined function and protease activity [49]. It is composed of N-terminal zinc finger repeats, followed by a conserved protease core domain (DI-II) and a C-terminal SOL homology domain [95]. To help better understand the physiological roles of these enzymes and to aid in potential drug discovery, it would be useful to have isoform-specific small-molecule inhibitors. In an attempt to develop such inhibitors, the active-site specificity must be characterized. This has previously been done for the protease cores of µ-calpain (µI-II) and a G203A mutant of the m-calpain protease core (mI-II G203A), where a peptidomimetic epoxide library of inhibitors was used to profile their active-site preferences [64]. The use of mini-calpains in biochemical characterization offers many advantages, including their ease of expression, resistance to autoproteolysis, and simpler crystallization compared to the full-length calpains. Here we have examined the protease cores of several calpain isoforms for their relative activity and profiled the active site of the tissue-specific calpain 8 using an epoxide library of inhibitors. 2.4 Materials and Methods 20 2.4.1 Materials Inactive rat m-calpain (mC105S) and the protease core of rat µ-calpain (µI-II) were expressed in E. coli and purified as previously described [37,44]. The triple-wide gel electrophoresis apparatus was obtained from C.B.S. Scientific (Solana Beach, CA). The positional-scanning epoxide libraries were synthesized as described elsewhere [96], and used from 50 mM stocks in 100 % DMSO. The structures and names of the nonnatural amino acids used are provided in the supplementary table to Cuerrier et al. 2007 [64]. The protease cores of calpains 3, 8, 9 and 15 were provided by the Structural Genomics Consortium (Toronto, ON). 2.4.2 Mutagenesis Site-directed mutagenesis using the QuikChange kit (Stratagene, CA) was performed on mini-calpain 8 to mutate Gly203 to Ala. The primers used for the mutation were 5' CCACAGTGGAGGCCTTTGAGGATTTCAC 3' (sense) and 5' GTGAAATCCTCAAAGGCCTCCACTGTGG 3' (anti-sense) with the Ala mutation (underlined in bold) that introduced a StuI cut site AGG/CCT for screening clones. 2.4.3 Relative Activity Measurements on Protease Cores A solution containing 1 mg/mL of inactive m-calpain (mC105S) and 1 mg/mL of calpain protease core (either calpain 3, 8 wild type, 9 or 15) was made in reducing buffer (50 mM HEPES-NaOH pH 7.8 and 10 mM DTT). Equal amounts of this solution and reducing buffer containing 100 mM CaCl2 and 5 % DMSO were used to initiate 21 digestion. The reaction proceeded at 30 °C and was quenched with 100 mM EDTA and 3X SDS-PAGE sample buffer (150 mM Tris-HCl (pH 6.8), 300 mM DTT, 6 % SDS, 0.3 % bromophenol blue, and 30 % glycerol) at 0, 0.5, 1, 3.5, 5, 8.5 and 25 h after initiation. A control reaction was run where equal amounts of reducing buffer were added in the absence of calcium. The samples were run on an SDS-PAGE gel to compare the extent and rate of digestion of the 80 kDa large subunit of mC105S. 2.4.4 Inhibitor Profiling Digestions were set up as above with the addition of 0.24 mM of the library of epoxide inhibitors with 19 natural and 42 non-natural amino acids at the P2 position with (R,R) or (S,S) stereochemistry. The reaction was incubated at 30 °C for 25 h and quenched with 100 mM EDTA and 3X SDS-PAGE sample buffer. A triple-wide gel apparatus was used to run up to 65 samples on a 12 % polyacrylamide gel. Densitometry analysis was done on the 80 kDa band using the free open source Image J software (http://rsb.info.nih.gov/ij/). The background intensity above each band was subtracted from every lane. The reactions were compared by assigning to each a preference equal to its fold-over average inhibition, the average being 1.0. 2.5 Results and Discussion 22 2.5.1 Mini-calpains 3, 8 and 9 are Less Active than µI-II Inactive m-calpain (mC105S) was used as a substrate for the protease cores to determine the activity of mini-calpains 3, 8, 9 and 15 relative to µI-II (Fig. 2.1). For mini-calpain 8, there are three protein bands present at zero time (Fig. 2.1B). Bands (i) and (vi) are the large and small subunits, respectively, of the substrate, and band (iv) is that of the mini-calpain enzyme. After 1 h, three digestion products appear. Band (ii), just below the 80 kDa band, contains domains I-III. A domain I-II fragment of the mC105S substrate appears just above that of mini-calpain (band iii). The other, just above the 21 kDa small subunit band, is domain IV- the other cleavage product (band v). These digestion products increase in intensity during the course of the digestion and do not appear in the absence of calcium (control data shown in Fig. 2.3). The extent of digestion of the substrate by mini-calpain 8 after 25 h is roughly half that seen after just 0.5 h when µI-II is used as the enzyme (Fig. 2.1A). The digests produced by mini-calpains 3 and 9 are qualitatively the same as that produced by mini-calpain 8 but they proceed at about one third of the rate seen with mini-calpain 8 (Fig. 2.1C and 2.1D, respectively). Indeed, the domain I-II digestion product is barely visible even after 25 h of digestion. In this assay, mini-calpain 15 was shown to be inactive over a 25 h period (Fig. 2.1E). The activity of mini-calpain 15 has not yet been determined and it may be that this isoform does not function as an enzyme, mC205S has no recognition sites for it, or that it requires the whole protein for activity [49]. 23 A 0 B 0 0.5 25 0.5 1 3.5 5 8.5 25 (I) (II) (III) (IV) (V) (VI) 80K 38K 21K C 0 0.5 1 D 0 3.5 5 8.5 25 E 0.5 1 3.5 5 8.5 25 0 0.5 1 3.5 5 8.5 25 Figure 2.1. Inactive m-calpain (mC105S) hydrolysis activity assay of mini-calpains 1 (A), 8 (B), 3 (C), 9 (D) and 15 (E). This assay monitors the digestion of the 80 kDa large subunit of mC105S (band i) by mini-calpain (band iv) into its cleavage products: Domains I-III (band ii), III (band iii), and IV (band v). Band vi represents the small subunit. Each digestion was done at 30 °C in the presence of 50 mM CaCl2 and 0.5 mg/mL of each mini-calpain and mC105S. Sample (10 µL) was added to 10 µL of SDS-PAGE loading buffer and loaded on a 12 % polyacrylamide gel. The time of digestion is listed in hours above each lane and the calcium controls are shown in Fig. 2.3. 24 2.5.2 Mutation of Mini-calpain 8 Increases its Activity In the protease core of rat m-calpain, G203 destabilizes part of helix α7 in the absence of the supporting domains III, IV and VI (small subunit) causing Trp 106 to swing into the active site, resulting in a decrease in activity (Fig. 2.2A) [97]. In fact, its activity is only 1 % of µI-II, which has an Ala at position 203, and Trp116 pointed away from the active site (Fig. 2.2B). The recent structure of calcium-bound full-length m-calpain displays the stabilization of this helix by the presence of DIII, DIV, and DVI, even with a Gly at this position (Fig. 2.2C). In an attempt to increase the activity of mini-calpain 8, the Gly at position 203 in the conserved helix α7 was mutated to an Ala. The activity was increased about two-fold, a much lower effect that was seen in mI-II (Fig. 2.3). A closer look at the structure (PDB entry 2NQA) revealed that the Trp at residue 106 was not protruding into the active site in the presence of the destabilized helix α7, as was seen with mI-II (Fig. 2.2D). This supports the smaller difference in activity upon mutation of G203 to an Ala seen with mini-calpain 8 than with mI-II. 2.5.3 Mini-calpain 8 has Preferences for Leu, Ile and Val at the S2 Subsite Of the tissue-specific mini-calpains, the core of calpain 8 was the only one with sufficient activity to attempt inhibitor profiling. Specificity at the P2 site was determined by profiling the digestion of mC105S by mini-calpain 8 with a peptidomimetic library of compounds, containing a fixed amino acid at the P2 position and an equimolar mix of natural residues at the P3 and P4 positions with both (R,R) and (S,S) stereochemistry at 25 A B C D Figure 2.2. The effect of the stability of α7-helix on Trp106 (purple) in mI-II (A), µI-II (B), m-calpain (C) and mini-calpain 8 (D). Leupeptin is shown in the active site of all 4 structures, Trp106 is coloured purple and position 203 is coloured yellow. (A) In the absence of supporting DIII-DVI, G203 of mI-II destablizes α7-helix, causing Trp106 to swing into the S2 pocket of the active site, decreasing its activity [97]. (B) Position 203 of µI-II is occupied by a helix-stabilizing Ala and Trp116 is positioned away from the active site. (C) In full-length m-calpain, the presence of DIII-DVI stabilize α7-helix even with G203, causing Trp106 to face away from the active site. (D) The protease core of calpain 8 has a Gly at this conserved position that disrupts α7-helix but does not cause Trp106 to swing directly in the active site, it is pushed downwards instead. The PDB entries are 1mdw, 1tl9, 1df0 and 2nqa, for the x-ray crystallographic structures of mI-II, µIII, m-calpain and mini-calpain 8, respectively. The protein backbone is coloured as shown in Fig. 1.1. 26 B A 0.5+ 0.5- 0 0.5 C 5 25 0 0.5 5 25 Figure 2.3. Autolysis assay of mini-calpain 8. The time points are listed in hours and the autolysis fragments are the same as shown in Fig. 2.1. (A) The control hydrolysis of mC105S (0.5 mg/mL) by µI-II (0.5 mg/mL) after 30 min with (+) and without (-) 50 mM CaCl2 at 30 °C. (B and C) The hydrolysis from 0 to 25 h for 0.5 mg/mL wild type (WT) (B) and mutant (G203A) mini-calpain 8 (C) at 30 °C. Sample (10 µL) was added to 10 µL of SDS-PAGE loading buffer and loaded on a 12 % polyacrylamide gel 27 the epoxide warhead (Fig. 2.4). SDS-PAGE shows differential digestion of the mC105S 80 kDa band by mini-calpain 8 into its cleavage products, as described in section 2.4.1 (Fig. 2.5). Using the library with (S,S) stereochemistry, a preference was demonstrated at the P2 site of the inhibitor for the branched aliphatic natural amino acids Leu, Ile and Val, and also a slight preference for Trp. This preference was similar to that observed with µIII (Fig. 2.6). The library with (R,R) stereochemistry showed similar preferences as the (S,S) library, although the difference is much smaller (Fig. 2.6). The non-natural amino acid profiles showed similar preferences for numbers 2, 8, 16, 18, 19, 23, 27 and 42 for the (S,S) library as previously determined for µI-II and mI-II [98], but failed to pull out preferences for the (R,R) library (Fig. 2.7). This demonstrates that mini-calpain 8 is calpain-like, as it exhibits similar preferences at the P2 site to both µI-II and mI-II. The preferences were much lower for mini-calpain 8 than for µI-II and mI-II, which may reflect its lower activity. In summary, the relative activity of the protease cores of calpains 3, 8, 9 were determined to be very low and statistical analysis was therefore not pursued. The low activity of the isoforms as well as the difficulty in profiling mini-calpain 8 stresses the need for the isolation of the full-length enzymes for inhibitor and substrate profiling. The whole enzymes are expected to have a higher activity as well as provide additional preferences at the unprimed side of the active site by the close association of domain III. 28 P4 P2 m ix HN O O X H N N H m ix O O N H (S,S) or (R,R) O Et O O P3 Figure 2.4. Structure of the peptidomimetic epoxide library. A fixed amino acid is at the P2 scanned position (X) and a mix of natural amino acids at the P3 and P4 sites. Natural amino acids except methionine and cysteine, with the addition of norleucine as well as the non-natural amino acids were scanned at the P2 position. The library contained both (S,S) and (R,R) stereochemistry at the epoxide warhead to increase the potential for specificity [64]. 29 A D L 1 2 9 N 3 10 E 4 11 F G H I K T V W L N P Q R S (+) (-) Y N.L Lep V W P Q R S I K T ( (-) Y N.L A D E F G H + ) 6 12 7 5 13 14 (-) 8 15 16 (+) 22 23 lep 24 18 17 25 19 26 20 27 21 28 30 29 31 38 32 39 33 40 34 (-) 36 35 37 (+) 42 41 Figure 2.5. SDS-PAGE of mC105S hydrolysis by mini-calpain 8. The hydrolysis of 0.5 mg/mL mC105S by 0.5 mg/mL mini-calpain 8 was done in the presence of a peptidomimetic library of epoxide inhibitors (0.24 mM) probing the S2 subsite of mini-calpain 8 with natural (top) and non-natural (bottom) amino acids with (R,R) (coloured red) and (S,S) stereochemistry at the epoxide warhead [(R,R) stereochemistry not shown for the non-natural amino acid gel]. The bands are as described in Fig. 2.1. SDS-PAGE loading buffer (5 µL) was added to 5 µL sample and loaded onto 12 % polyacrylamide gel. The gel was stained with Coomassie Blue. (+) no inhibitor; (-) no calcium; N.L norleucine; Lep leupeptin. 30 A Residue Preference 2.5 2.0 1.5 1.0 0.5 0.0 A D E F G H I K L N P Q R S T V W Y N.L Amino Acid B 3.0 Residue Preference 2.5 2.0 1.5 1.0 0.5 0.0 A D E F G H I K L N P Q R S T V W Y N.L Amino Acid Figure 2.6. Profiling the S2 subsite of mini-calpain 8 with the natural amino acids. Inhibitor profiles of the P2 (R,R) (A) and (S,S) (B) natural amino acid library with mini-calpain 8 (top) compared to µI-II (bottom left) and mI-II G203A (bottom right) [64]. Residue preference = foldover average inhibition where average is equal to 1.0 (black line). Error bars correspond to +/one standard deviation based on three separate experiments. 31 A 1.4 Residue Preference 1.6 1.2 1.0 0.8 0.6 0.4 0.2 0.0 1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 41 Amino Acid Residue Preference B 2.0 1.8 1.6 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 41 Amino Acid Figure 2.7. Profiling the S2 subsite of mini-calpain 8 with the non-natural amino acids. Inhibitor profiles of the P2 (R,R) (A) and (S,S) (B) non-natural amino acid library with minicalpain 8 (top) compared to µI-II (bottom left) and mI-II G203A (bottom right) [64]. Residue preference = fold-over average inhibition where average is equal to 1.0 (black line). Error bars correspond to +/- one standard deviation based on three separate experiments. 32 Chapter 3 Profiling of calpain activity with a series of FRET-based Substrates 3.1 Preface This chapter has been submitted to FEBS letters. Kelly, J.C., Cuerrier, D., Campbell, R.L., and Davies, P. (2008). Profiling of calpain activity with a series of FRET-based substrates. FEBS Letters. Submitted. Jacqueline Kelly was responsible for completing the digestion and mass spectrometry analysis to confirm the cleavage site of PLFMER. Dr. Dominic Cuerrier profiled the alanine scanning variants of PLFAER with µI-II, which was repeated by Jacqueline who extended this approach to the full-length µ- and m-calpains. The experiments were performed under the guidance of Dr. Peter L. Davies with advice from Dr. Robert L. Campbell and Dr. Dominic Cuerrier. Most of the alanine variants were synthesized by the Alberta Peptide Institute (Edmonton, Alberta) and the others were synthesized in-house. Mass spectrometry was performed by Dr. YiMin She (Queen’s University, Kingston, Ontario). The manuscript was written by both Jacqueline Kelly and Dominic Cuerrier with guidance from Dr. Peter L. Davies with editorial input from Dr. Robert L. Campbell. 33 3.2 Abstract Calpains are intracellular proteases that selectively cleave proteins in response to calcium signals. Although calpains cut many different sequences, residue preferences within peptide substrates were recently determined and incorporated into a superior FRET-based substrate (PLFAER). Here we show PLFAER is cleaved by calpain at the intended F-A scissile bond. Sequential replacement of individual residues by alanine reduced activity except with PLFAAR, which is cleaved 2.3 times faster than PLFAER. The rates of hydrolysis of the alanine-substituted substrates were used to compare calpain substrate preferences. These profiles for full-length µ- and m-calpains were similar and closely matched that of the protease core of calpain 1. 3.3 Introduction Calpains are intracellular cysteine proteases that are activated by calcium to make discrete cuts in a wide variety of protein substrates as directed by calcium signaling. Physiological roles for these select cleavages include the promotion of cell motility [15], cell division [16], granule secretion [99] and apoptosis [17]. Many of the welldocumented calpain substrates are cytoskeletal proteins such as MAP2 [100], tau [101], talin [102], spectrin, integrin [103] and vimentin [14]. Compilations of their cut sites reveal very little sequence specificity other than a preference for a hydrophobic residue (particularly Leu) in the P2 position [63,104,105]. However, it is also true that little attention has been paid to the issue of how quickly individual sites are cleaved. Thus the consensus sequence compiled by Tompa et al. includes all documented protein cleavage 34 sites irrespective of whether they are cut rapidly or slowly [100]. As with any protease, cleavage of folded proteins is limited to discrete unstructured sections that generally occur in loops, linker regions and termini. This might account for the abundance of proline in the consensus sequence, since this amino acid disrupts secondary structure. In view of these limitations, we earlier examined the sequence specificity of calpain by experimentation [63] using the degenerate peptide library method pioneered by Turk et al. (2001) [65]. This showed that there were preferred residues at the P1' (Met or Ala), P2' (Glu) and P3' (Arg) positions in calpain peptide substrates. The incorporation of this primed side sequence into a second degenerate peptide library led to the identification of optimal residues at the unprimed positions. These were primarily aliphatic and aromatic residues, including Leu at P2. The combination of preferences gave rise to a hexapeptide Pro-Leu-Phe-Met/Ala-Glu-Arg sequence that was incorporated into a FRET substrate with an N-terminal EDANS donor group and a C-terminal DABCYL quencher. When this was compared to other hexapeptide FRET substrates, it was found to be cleaved by the protease core of µ-calpain eight times more rapidly than the consensus cleavage sequence Pro-Leu-Lys-Ser-Pro-Pro and 18 times more rapidly than the spectrin cleavage sequence Glu-Val-Tyr-Gly-Met-Met [63]. The superiority of PLFAER as a calpain substrate was confirmed by Polster et al. who compared it to other known calpain substrate sequences [106]. This group used the increased specificity of PLFAER to produce a biomarker derivative that has improved cell permeability. Through slight modifications, they also reduced its digestibility by trypsin and chymotrypsin. 35 In the present study, we set out to further characterize this kinetically optimized substrate. We validated the preferred substrate sequence by confirming its cleavage at the intended scissile bond between Phe and Met. Confirmation of the optimal fit of each residue was performed by replacing them in turn with alanine, resulting in the discovery that the sequence Pro-Leu-Phe-Ala-Ala-Arg is an even better substrate for the calpain protease core (µI-II). Lastly, we used this set of FRET peptides to show that µ- and mcalpain have similar substrate preferences and that the protease core of µ-calpain has only slightly different substrate preferences to those of the whole enzyme. 3.4 Materials and Methods 3.4.1 Materials The FRET substrates, (EDANS)-EPLFAERK-(DABCYL), (EDANS)EPLAAERK-(DABCYL), (EDANS)-EPAFAERK-(DABCYL), (EDANS)-EALFAERK(DABCYL), (EDANS)-EPLFMERK-(DABCYL), and (EDANS)-EPLFGERK(DABCYL) were synthesized by the Alberta Peptide Institute (Edmonton, Alberta). The FRET substrates (EDANS)-EPLFAARK-(DABCYL) and (EDANS)-EPLFAEAK(DABCYL) were synthesized in-house. Bold type indicates the residue substituted into the original (EDANS)-EPLFAERK-(DABCYL). µ-Calpain was purchased from Calbiochem (cat# 208712). The recombinant protease core (µI-II), and m-calpain were prepared as described previously [37,44]. 36 3.4.2 Determination of the Cleavage Site within the PLFAER Substrate The substrate (EDANS)-EPLFMERK-(DABCYL) was incubated at a concentration of 50 µM with 3.2 µM µI-II in cleavage buffer (50 mM MOPS pH 7.6, 100 mM NaCl, 10 mM CaCl2, 0.1% β-ME) and allowed to react to completion. Similarly, an undigested control reaction in Ca2+-free conditions was prepared in 50 µM (EDANS)EPLFMERK-(DABCYL) and 3.2 µM µI-II in control buffer (50 mM MOPS pH 7.6, 100 mM NaCl, 1 mM EDTA and 0.1% β-ME). Digestion was monitored by a Perkin Elmer LS50B luminescence spectrometer with excitation and emission wavelengths of 335 nm and 500 nm, respectively. The reaction was allowed to go to completion, until no further increase in fluorescence was seen, and the sample was flash frozen with ethanol/dry ice and stored at -20 °C. Both samples were analysed on an Applied Biosystems/MDS Sciex QSTAR XL QqTOF mass spectrometer with a nano-ESI source. 3.4.3 Effect of Individual Residue Substitutions on Cleavage Kinetics using µI-II, µand m-calpain The rate of hydrolysis of the FRET substrates by µI-II was monitored by fluorescence in a 1 mL cuvette as described above. Readings were obtained at 1 s intervals. The cuvette contained 2, 5 or 10 µM substrate, 0.71 µM µI-II in 50mM Tris-HCl pH 7.6 and 10 mM DTT and the reaction was initiated by 5 mM CaCl2 in a final volume of 1 mL. The amount of substrate digested per relative fluorescence unit (RFU) was calculated for each individual substrate by using a control that was digested to completion with papain. Briefly, substrate (2 µM) in control buffer was analyzed in the fluorimeter with and 37 without 0.35 µM papain and the change in fluorescence per µMole substrate was calculated (Equation 1). Fluorescence yield per µM = (RFUdigested – RFUundigested)/2µM Equation 1 These values were then used to calculate initial rates of hydrolysis by calpain and were normalized relative to the initial rate of hydrolysis of PLFAER at the same substrate concentration (all reactions were performed in the linear portion of the Michaelis-Menten curve, where [S] << Km). A correction factor for the inner filter effect was also incorporated as shown below. This experiment was repeated with µ- and m-calpain with the exception that 0.13 µM enzyme was used for both and 1 mM CaCl2 was used with µcalpain. The inner filter effect for the 1 mL cuvette in the Perkin Elmer LS50B luminescence spectrometer was corrected for by determining the change in fluorescence caused by 0.5 µM EDANS FRET donor at each substrate concentration as described previously by Liu et al., 1999 [107] . Fluorescence was monitored at excitation and emission wavelengths of 335 nm and 500 nm respectively for PLFAER at 0, 0.5, 1, 2, 5, 8 and 10 µM in buffer 3. EDANS (0.5 µM) was then added and the change in fluorescence was determined for each substrate concentration. A correction factor was calculated for each individual substrate and applied to the rates of hydrolysis (Equation 2). 38 Correction = RFUEDANS (at each substrate concentration) Equation 2 RFUEDANS (in the absence of substrate) 3.5 Results and Discussion 3.5.1 Determination of the Substrate Cleavage Site The FRET substrate (EDANS)-EPLFMERK-(DABCYL) was hydrolyzed by the calpain protease core, µI-II, until there was no further increase in fluorescence. The digestion products were analyzed by MALDI-MS together with an undigested control (Fig. 3.1). The control demonstrated a peak at m/z 775 corresponding to the uncleaved FRET substrate with a +2 charge. There were no detectable signals at m/z values corresponding to potential cleavage products. In the digested sample, there was no sign of the substrate peak at m/z 775 (+2 charge), which indicates that the digestion had gone to completion. Instead, a primary cleavage site of the substrate between F and M was demonstrated by peaks at m/z 753 (EDANS-EPLF, +1), 377 (EDANS-EPLF, +2), 408 (MERK-DABCYL, +2) and 272 (MERK-DABCYL, +3) (Fig. 3.1). A minor peak with m/z 342 corresponds to ERK-DABCYL, representing a secondary cleavage site between M and E at the P1' and P2' positions, respectively. The absence of a peak corresponding to (EDANS)-EPLFM identifies this cleavage as secondary to the P1-P1' hydrolysis. The [M+H]+ values of the observed peaks correspond well with the theoretical values with similar systematic errors (Table 3.1). The primary cleavage site revealed through mass 39 407.693 1322 1200 272.132 1000 800 342.176 600 256.135 400 200 0 753.312 377.164 400 600 800 m/z, amu 1000 Figure 3.1. NanoESI-MS spectrum of the cleavage products from the hydrolysis of (EDANS)-EPLFMERK-(DABCYL) by µI-II. Peaks at m/z of 753.3 and 377.2 represent the (EDANS)-EPLF product with a +1 and +2 charge respectively. Mass to charge ratios of 407.7 and 272.1 indicate the c-terminal end of the substrate (MERK-(DABCYL)) with a +2 and +3 charge respectively. A secondary cleavage is also shown between the P1' Met and P2' Glu at a m/z of 342.2 40 Potential Hydrolysis Product (EDANS)-E Observed [M+H]+ (ion state) ∆[M+H]+ 683.362 683.352 (+2) 0.01 753.328 753.320 (+1, +2) 0.008 814.403 814.391 (+2, +3) 0.011 Theoretical [M+H]+ 396.122 K-(DABCYL) (EDANS)-EP 398.219 493.175 RK-(DABCYL) (EDANS)-EPL 554.320 606.259 ERK-(DABCYL) (EDANS)-EPLF MERK-(DABCYL) (EDANS)-EPLFM FMERK-(DABCYL) (EDANS)-EPLFME LFMERK-(DABCYL) (EDANS)-EPLFMER PLFMERK-(DABCYL) (EDANS)-EPLFMERK-(DABCYL) 884.368 961.471 1013.411 1074.555 1169.512 1171.608 1548.713 Table 3.1. Potential and observed FRET substrate hydrolysis products. The FRET substrate (EDANS)-EPLFMERK-(DABCYL) was fully digested by µI-II in the presence of 10 mM CaCl2 and analysed by Nano.ESI-MS. All product masses are represented in their mono-protonated form ([M+H]+ and the systematic error between the observed and theoretical [M+H]+ values is shown (∆[M+H]+). 41 spectrometry between F and M supports the inferred position of the scissile bond obtained through substrate profiling [63]. 3.5.2 Efficacy of Ala and Met at the P1΄ Position The peptide sequence PLFMER is rapidly cleaved by µI-II at the F-M bond, but the extent to which each residue contributes to substrate recognition and binding in the context of the whole peptide is unknown. Although it is assumed that each side-chain makes a positive contribution to the reaction based on previous research [63], this claim has been tested here. We initially looked at the preference between Met and Ala at the P1' position, where these two residues were strongly favoured in the study by Cuerrier et al., 2005 [63]. Although Met had been selected over Ala in a ratio of 4:3, in the context of the whole peptide (Table 3.2), it was found to have only a slightly higher (though not statistically significant) rate of hydrolysis compared to PLFAER (Fig. 3.2). The latter substrate is preferred over the former for routine assays because Met is susceptible to oxidation. PLFMER, however, was used in the mass spectrometry analysis of the preferred cleavage site because the mass differences in the expected cleavage products were more distinct with Met in the P1' position. 3.5.3 Effect of Individual Residue Substitutions on Cleavage Kinetics To evaluate the contribution of specific residues in the other positions of the optimal sequence, a series of alanine-substituted FRET substrates was synthesized. For the P1' position already represented by alanine, a glycine substitution was made. The rate 42 Amino Acid Selectivity Ratio P1’ P2’ P3’ M 4.05+/-0.47 2.34+/-0.24 1.56+/-0.31 A 2.84+/-0.35 1.94+/-0.31 0.91+/-0.06 E 0.74+/-0.13 2.13+/-0.57 0.35+/-0.29 R 2.39+/-0.02 0.91+/-0.16 2.91+/-0.77 G 0.33+/-0.04 0.67+/-0.30 0.47+/-0.16 Table 3.2. Preference for each amino acid at P1'-P3' positions of the hexapeptide substrate. The abundance of Met (M), Ala (A), Glu (E), Arg (R) and Gly (G) at the P1’, P2’ and P3’ positions of the hexapeptide substrate, using a substrate library approach, shown as a selectivity ratio. The selectivity ratio is defined as the ratio of the abundance of the residue observed at the position to the abundance of the residue expected in the absence of any specificity (For more information on the methods see [63]). 43 of the hydrolysis of these substrates by µI-II, µ- and m-calpain was determined and compared to the unsubstituted PLFAER substrate. The PLFAER sequence was designed from data obtained using µI-II [63]. With this protease construct, alanine-substitutions of the P2-Leu and the P1-Phe had the greatest detrimental effect on substrate hydrolysis, with corresponding substrates demonstrating 4 % and 8 % of the rate of hydrolysis of the unsubstituted substrate, respectively (Fig. 3.2A). This is consistent with these two subsites interacting with a large hydrophobic surface area in the substrate binding pocket, as observed from the P2-Leu P1-Phe sequence of inhibitor SNJ-1945 in complex with µIII [62], and highlights the importance of these two positions on cleavage kinetics. The P3'-Arg to Ala substitution reduced the rate of hydrolysis to 20 %, demonstrating the importance of this relatively distal primed-side position. Substitution of the P1'-Ala by Gly showed a similar detrimental effect (28 %), possibly by increasing the conformational flexibility of the peptide. Alanine-substitution of the P3-Pro only had a minor impact (73 %). Nevertheless, the presence of the P3-P1' and the P3' residues of the unsubstituted substrate were all preferred over their Ala or Gly replacements, confirming the positive effect of the side-chains. Interestingly, the P2'-Glu to Ala substitution improved the cleavage of the substrate (2.3-fold), suggesting that Ala is preferred at this position in the context of the whole peptide. In retrospect this result is not in conflict with the original specificity profiles [63], as the P2' position demonstrated selectivity for Ala and Met in addition to Glu (Table 3.2). The reason Glu was incorporated at the P2' position was because the 44 A ALFAER --PAFAER --PLAAER --PLFAER --PLFAAR --PLFAEA --PLFGER --PLFMER --0 1 2 3 Preference B ALFAER --PAFAER --PLAAER --PLFAER --PLFAAR --PLFAEA --PLFGER --PLFMER --0 1 2 Preference C ALFAER --PAFAER --PLAAER --PLFAER --PLFAAR --PLFAEA --PLFGER --PLFMER --0 1 2 Preference Figure 3.2. Relative initial rates of the hydrolysis of the substituted FRET substrate series by µI-II (A), µ-calpain (B) and m-calpain (C) normalized relative to PLFAER (preference =1.0). Each substrate was analyzed in duplicate for µ-calpain, and triplicate for µI-II and mcalpain at 2, 5 and 10 µM. The internal peptide sequence of the substrates are shown with the substituted amino acid underlined, red and in bold. The peptide with the internal sequence PLFAER corresponds to the unsubstituted substrate. Error bars correspond to +/- one standard deviation. 45 presence of Ala and Met in the second cycle was misinterpreted as carry over from the first cycle of the Edman degradation reaction, possibly due to incomplete coupling or cleavage [63]. Therefore, contrary to initial expectations, the sequence PLFAAR is cleaved more rapidly, and corresponds to a better calpain substrate than the sequence PLFAER. Using the panel of alanine-substituted sequences, the substrate cleavage profiles for µ- and m-calpain were found to be very similar (Fig. 3.2). Moreover, they are qualitatively similar to that of µI-II with a few distinctions. The P3-Pro to Ala substitution appears to have slightly improved rates of hydrolysis by the full-length calpains (1.4- and 1.1-fold for µ- and m-calpain respectively compared to 0.73-fold with µI-II). This may be attributed to the added rigidity provided by the proline, which may force the Glu and EDANS fluorophore into a less favourable position within domain III that flanks the active site. Since the active site of µI-II is open on the unprimed side due to the lack of domain III, it is less restrictive at this position. Also, the P1'-Ala to Gly substitution is not as detrimental in the full-length enzyme as was shown with µI-II (1.2and 0.71-fold for µ- and m-calpain respectively, compared to 0.28-fold with µI-II). It has been suggested that the active-site conformation of µI-II is more labile than in full-length calpain because it lacks the support of the other domains. Indeed, the crystallization of calpain protease cores is facilitated by having covalently bound inhibitors in the active site cleft to make stabilizing contacts with the flanking domains I and II [43,62,108]. Therefore, the full-length enzyme is likely to tolerate the loss of a side chain in P1' better 46 than the less stable protease core µI-II. The P2'-Glu to Ala substitution was found to produce a higher rate of hydrolysis compared to the original substrate with both µ- and m-calpain as well as with µI-II. Thus, PLFAAR is a superior substrate sequence for the whole enzyme of both major calpain isoforms as well as the protease core. Now that we have improved the PLFAER substrate that already showed superiority to other synthesized substrates, PLFAAR can be stabilized as described by Polster et al. and tested as a biomarker for calpain activity in cells [106]. In summary, we have demonstrated by mass spectrometry that the FRET substrate, (EDANS)-EPLFMERK-(DABCYL), is cleaved by µI-II between the F and M residues. Alanine-scanning variants were used to validate the importance of the P3-Pro, P2-Leu, P1-Phe and P3'-Arg. Also, an improved sequence, PLFAAR, was determined to have a 2.3-fold increase in initial rates of hydrolysis by µI-II relative to the original PLFAER sequence. Full-length µ- and m-calpain were profiled with these alaninescanning variants, for comparison with the protease core profile. The profiling shows that the two major calpains have very similar substrate preferences. In addition, with two minor exceptions, these substrate preferences are retained in the protease core that lacks the support of domains II, IV, V and VI. Profiling with these FRET substrates can potentially be extended to the other calpain isoforms. These non-classical calpains may exhibit different active-site specificities, which can be used in the development of isoform-specific calpain inhibitors. 47 Chapter 4 The Stabilization of Calcium-bound Calpain by Calpastatin Peptides 4.1 Preface This chapter will form the basis of a manuscript on the stabilization of calcium-bound calpain. Kelly, J., Hanna, R., Davies, P.L. (2008). The stabilization of calcium-bound calpain by calpastatin peptides. Jacqueline Kelly was responsible for cloning and purification of the CAST C-A peptides as well as the enzyme and gel autolysis assays. The turbidity assays were done with assistance from Rachel Hanna. The concentrations of the CAST C-A peptides were determined by amino acid analysis performed by The Advanced Protein Technology Centre (Hospital for Sick Children, Toronto, ON). The experiments were performed under the supervision and guidance of Dr. Peter L. Davies with helpful input from Rachel Hanna. The chapter was written by Jacqueline Kelly with insight from Dr. Peter L. Davies. 48 4.2 Abstract Calpains are non-lysosomal cysteine proteases activated by intracellular influxes of calcium. Over-activation of the calpains by misregulated calcium levels contributes to many pathological conditions and is one reason to develop pharmacological inhibitors of these enzymes. To avoid problems with autolysis and calcium-induced precipitation that hamper work with the full-length enzymes, this has previously been pursued with the protease cores of calpain. However, they are much less active than the full-length enzymes and are missing a domain that contributes to inhibitor specificity. Here we have explored the use of segments of the natural inhibitor, calpastatin, to prevent calciuminduced calpain precipitation. Peptides that span the three different, contiguous C and A sub-domains of calpastatin, while omitting the inhibitory B sub-domain, were made and mixed with calpain at different ratios. All three C-A peptides inhibited precipitation at a 2.5:1 molar ratio of peptide to enzyme as monitored by optical density at 320 nm. Peptide 3C-4A was the most effective of the three and prevented the onset of precipitation even at a 1:1 molar ratio. In addition, the peptides did not affect the activity of calpain or its inhibition by leupeptin. Although the peptides are cleaved by active m-calpain, they continue to protect the enzyme from aggregation and precipitation. This method increases the opportunities for using full-length calpains in in vitro experiments such as X-ray crystallography and active-site profiling of substrates and inhibitors. 49 4.3 Introduction Calpains are a family of cysteine proteases that perform limited proteolysis of their target substrates in response to increases in intracellular calcium levels [14]. Since calpains become unregulated and over-activated in many disorders such as reperfusion injury following stroke and heart attack, Parkinson’s disease and Duchenne muscular dystrophy, they are key targets for the development of small-molecule inhibitors [109]. One obstacle to the use of calpains in drug development has been their instability and tendency to aggregate when their cofactor, calcium, is present. This problem has partly been resolved with the use of the protease core (DI-II) of calpain, which is stable and active in the presence of calcium [44]. The protease core of µ-calpain (µI-II) has been used to determine optimal inhibitor and substrate sequences at the active site [64,63]. Unfortunately, the protease cores are much less active than the full-length calpains. For example, µI-II is only 2-3 % as active as the whole enzyme [97]. They also lack the additional specificity provided by DIII at the unprimed side of the active site. Therefore, the stabilization of full-length calpains would allow one to work with the whole enzymes in the presence of calcium and may accelerate the development of calpain-specific substrates and inhibitors. Calpastatin is the only known inhibitor that is specific for the heterodimeric calpains. It can interact with up to four calpain molecules at one time [87] because it contains four homologous tandemly arranged inhibitory domains (CAST1-4). Each CAST domain interacts with calcium-bound calpain through three well conserved sub-domains: A, B 50 and C (Fig. 4.1A) [75,83,84,110] . Recently, calcium-bound m-calpain was cocrystallized in complex with CAST4 (Fig. 4.1B) [46]. The X-ray crystal structure of the complex revealed an aligned active site where sub-domain B of CAST bound, escaping cleavage by looping out around the active-site cysteine, and exerting its inhibitory affect by blocking access to substrates. This structure also confirmed the presence of small hydrophobic patches exposed upon calcium binding in DIV of the large subunit and DVI of the small subunit. CAST sub-domains A and C form amphipathic α-helices and make hydrophobic contacts with these regions in DIV and DVI, respectively [46,85,86,111]. It has been proposed by other researchers that the instability of calcium-bound calpain may involve the dissociation of the small subunit, leading to aggregation [112,70]. If so, it is possible that CAST helps stabilize calpain because it physically links the two subunits together. It is also possible that the exposed hydrophobic patches formed during the activation of calpain by calcium might promote aggregation when they are not bound and covered by CAST [46]. In the present study, we propose a mechanism by which calpain can be stabilized in the presence of calcium using a portion of its natural inhibitor while keeping the active site clear (Fig. 4.1C). The inter-domain regions of calpastatin that link the A and C sub-domains of CAST from one domain to the next (1C2A, 2C-3A and 3C-4A) were recombinantly produced to provide a physical link between the PEF domains (DIV and DVI) (Fig. 4.1A). Although the dissociation constants between calpain and each CAST domain have previously been determined by surface plasmon resonance, the C-A linkers between each domain are hybrid molecules and have 51 A L 1 2 3 4 A B C A B C A B C A B C 86 64 98 C B B N C C C C A A N Figure 4.1. The proposed mechanism of calpain stabilization using CAST. (A) The domain structure of CAST including an N-terminal L domain followed by 4 homologous domains each consisting of A, B and C sub-domains. The regions highlighted with a red rectangle are the C-A peptides that were cloned to stabilize calpain and the number of amino acids for each peptide is written below. (B) The x-ray crystallographic structure of CAST bound to m-calpain. CAST is shown in purple with cylindrical helices and labelled sub-domains. The dashed line corresponds to floppy regions not resolved in the x-ray structure [46] (C) A model of the proposed mechanism of stabilization by the CAST C-A peptides. The active site that is normally occupied by the B sub-domain of CAST is free in this model. N- and C-termini of CAST are shown. 52 not been assessed in this way [87]. Therefore, each C-A peptide was tested for its degree of calpain stabilization. 4.4 Materials and Methods 4.4.1 CAST C-A Peptide Cloning DNAs coding for the CAST C-A peptides, 1C-2A, 2C-3A and 3C-4A, (Fig. 4.1) were amplified by polymerase chain reactions (PCR) using the following primers: CATTGCATATGATGGGAATCGACCATGCTATAG (sense 1C-2A); TCGATCTCGAGGTCTGGCTGCCGGGTGC (anti-sense 1C-2A); CATTGCATATGTGCCTGAGTGAGTCAGAGC (sense 2C-3A); TCGATCTCGAGTTCTGGATCTTCTTTCCTTGTC (anti-sense 2C-3A); CATTGCATATGGAACAACTTCCACCCTTAAGCG (sense 3C-4A); TCGATCTCGAGTGGTTTGTTCTCATCTGGATCAG (anti-sense 3C-4A). The DNAs coding for CAST 2C-3A and the pET24a vector (Novagen) were digested with NdeI and XhoI and directly ligated such that the expressed protein has a C-terminal His tag. The DNAs coding for CAST 1C-2A and 3C-4A were TA cloned into Top 10 cells (Invitrogen), released from the intermediate vector by digestion with NdeI and XhoI, gel purified and ligated into the NdeI and XhoI cut sites of the pET24a vector (Novagen). 53 4.4.2 Protein Expression and Purification Recombinant calpains, both active m-calpain (m80k/21k) as well as inactive (C105S 80k/21k) m-calpain, with their N-terminally truncated small subunit were expressed and purified as previously described [37,113]. CAST C-A plasmids were transfected into Escherichia coli BL21 (DE3) cells (Novagen) by electroporation. A single colony was used to inoculate LB media (25 mL) with kanamycin selection for growth overnight before starting a 1 L culture with LB/kanamycin broth. The cells were grown to an OD 595 nm of 1.0 at 37 °C. CAST C-A expression was initiated with 0.4 mM IPTG for 3 h. The cells were resuspended in lysis buffer (25 mM Tris-HCl pH 7.6, 5 mM EDTA, 5 % glycerol, 10 mM β-mercaptoethanol, 100 µM PMSF) and lysed via sonication. The supernatant was heated to 85 °C for 15 min and the insoluble particulates of denatured protein were removed by centrifugation. The soluble fraction was loaded on a 5-mL Ni2+-chelating agarose resin column (Qiagen). The column was washed with two column volumes of 25 mM Tris-HCl pH 7.6, 2 % glycerol, 500 mM NaCl buffer and eluted with 250 mM imidizole in the same buffer. The elution fractions containing the CAST C-A peptides were identified by SDS-PAGE, concentrated and flash frozen. 4.4.3 CAST C-A Peptide Amino Acid Analysis The CAST peptides have unusual amino acid compositions and two of the three peptides do not absorb at 280 nm. For these reasons, the concentrations of each peptide were determined using amino acid analysis. The Advanced Protein Technology Centre (Hospital for Sick Children, Toronto, ON) performed the analyses. 54 4.4.4 Monitoring Aggregation of mC105S by Turbidity The rate and extent of protein aggregation were monitored by optical density at 320 nm in a 96-well non-binding surface plate (Corning). The wells contained 4 µM mC105S with a 1-, 1.25-, 2.5- and 5-times molar ratio of CAST C-A peptides (4, 5, 10, and 20 µM, respectively) in 50 mM Tris-HCl pH 7.6, 10 mM DTT. Lastly, CaCl2 was added to a final concentration of 4 mM. The plate was shaken in a Powerwave XS Microplate spectrophotometer (Bio-Tek Instruments) for 10 s prior to each reading taken at intervals over 2 h at room temperature. Controls lacking calcium and CAST C-A were also done as described above. 4.4.5 The Effect of CAST C-A Peptides on m-Calpain Activity The hydrolysis of 10 µM (EDANS)-EPLFAER-(DABCYL) by 0.2 µM m-calpain was monitored on a Perkin Elmer LS50B luminescence spectrometer in the presence of 2 µM CAST C-A peptide in buffer containing 50 mM Tris-HCl pH 7.6, 10 mM DTT, and 4 mM CaCl2. The excitation and emission wavelengths were 335 and 500 nm, respectively. A calpain control in the absence of CAST C-A was also done. The digestion was repeated as described above with the addition of 0.5 mM leupeptin 3 min after initiation of the reaction. 4.4.6 The Effect of CAST C-A Peptides on m-Calpain Autolysis Autolysis of m-calpain in the presence of calcium with or without leupeptin was monitored by SDS–PAGE. A solution of 9 µM m-calpain in autolysis buffer (50 mM 55 HEPES, 100 mM NaCl, and 20 mM DTT) and 25 µM CAST C-A was added to an equal volume of calcium buffer (autolysis buffer + 10 mM CaCl2) and incubated at room temperature. Aliquots (20 µL) were taken at 0, 1.5, 3, 5, 10, 15, and 30 min and added to 20 µL 3X SDS-PAGE sample buffer (150 mM Tris-HCl pH 6.8, 300 mM DTT, 6 % SDS, 0.3 % bromophenol blue, and 30 % glycerol). The samples were heated at 95 °C and 10 µL was loaded on a Tris-tricine SDS 12 % polyacrylamide gel. 4.5 Results 4.5.1 Purification and Validation of the C-A Peptides The first step in the purification of the CAST C-A peptides was the heat denaturation of most E. coli proteins (Fig. 4.2A, lanes 1, 2). This step was very efficient as the C-A peptides lack secondary structure and are extremely heat stable, while most other contaminants denatured, aggregated and were removed by centrifugation. The soluble portion following heat treatment was subject to a Ni2+-affinity column, since the C-A peptides were cloned with a C-terminal His-tag (Fig. 4.2B). Most of the remaining contaminants were removed in the flow through and column washes (Fig. 4.2B, lanes 24) resulting in the elution of the CAST 1C-2A peptides (Fig. 4.2B, lanes 5-9). The expression and purification from 1 L of culture, yielded 20 mg, 22 mg, and 10 mg of CAST 1C-2A, 2C-3A and 3C-4A, respectively, and the concentrated samples of each CA peptide were shown to be >95 % pure based on SDS-PAGE (Fig. 4.2C). The expected molecular weights for CAST 1C-2A, 2C-3A, and 3C-4A were 9535, 7169, and 10823, 56 A 1 2 kDa 66 27 20 B M 2 3 4 5 6 7 8 9 kDa 66 43 35 27 20 14 6.5 C kDa 66 43 35 27 20 M 2 3 4 14 6.5 Figure 4.2. Purification of CAST 1C-2A. Lane M refers to the molecular weight markers. For (A) and (B), 10 µL of sample was added to 10 µL of SDS-PAGE loading buffer and 10 µL was run on a 12 % polyacrylamide SDS-PAGE gel. (A) Lane 1, cell lysate; Lane 2, supernatant of heated sample. (B) Ni2+-affinity Column. Lane 2, flow through of Ni2+ column; Lanes 3 and 4, Ni2+ column washes; Lanes 5-9, Ni2+ column eluted fractions. (C) Concentrated peptides (1 µg). Lane 2, CAST 1C-2A; Lane 3, CAST 2C-3A; Lane 4, CAST 3C-4A. 57 respectively, and were measured to be about 11000, 9000, and 14000, respectively by SDS-PAGE. The concentrated C-A peptides were sent for amino acid analysis to the Advanced Protein Technology Centre (Hospital for Sick Children, Toronto, Ontario). The number of each amino acid in the peptides was calculated using the DNA sequences and compared to the observed values based on the amino acid analysis (Table 4.1). This was done to validate the concentration obtained by this method and as a check on purity. The amino acid analyses of Ser, Met, and Lys were shown to be outliers based on the expected values and were not included in the calculated concentration. The His-affinity tag is represented in the amino acid analysis as six His residues. 4.5.2 CAST C-A Peptides Inhibit the Ca2+-Induced Insoluble Aggregation of mC105S To test the effect of the CAST C-A peptides on the stability of calpain without the added complication of autolysis, the inactive m-calpain that has the active-site cysteine mutated to a serine (mC105S) was used. The time of onset and rate of insoluble aggregation of mC105S were monitored by turbidity at 320 nm (Fig. 4.3). In the absence of calcium and CAST, there was no increase in OD 320 nm during the 200 min of the experiment (Fig. 4.3A). In the presence of calcium, aggregation occurred that was visible to the eye and was plotted as an increase in turbidity. The onset was rapid and turbidity reached a peak value after 15 - 20 min. The reversibility of this aggregation was shown 58 Amino Acid Ala Asx Glx Ser Gly His Arg Thr Pro Tyr Val Met Ile Leu Phe Lys Cys Trp Total 1C-2A 2C-3A 3C-4A Expected Observed Expected Observed Expected Observed 7 7.0 5 5.3 9 8.8 7 6.6 4 3.9 15 15.5 13 13.4 8 10.5 12 12.6 10 8.3 5 4.2 10 8.0 6 6.1 3 3.6 3 3.4 7 6.7 6 5.8 7 8.3 3 3.2 2 2.2 3 3.9 6 5.3 3 2.8 3 3.2 4 4.3 5 5.2 12 12.2 0 0.2 1 1.2 0 0.1 4 4.8 4 4.5 3 3.5 3 1.4 2 1.2 1 0.3 2 1.7 2 1.9 1 0.8 5 5.2 6 5.6 13 11.8 1 0.8 1 0.8 3 2.1 7 3.5 6 2.0 3 2.1 1 0.1 1 0.0 0 N/A 0 N/A 0 N/A 0 N/A 86 78.7 64 60.9 98 96.4 Table 4.1. Validation of the CAST C-A amino acid analysis. The number of each amino acid in the three C-A peptides as expected from the DNA sequence and observed from the amino acid analysis. 59 A + EDTA Optical Densitry (320nm) 0.7 0.6 0.5 0.4 Calcium Control 0.3 No Calcium Control 0.2 0.1 0.0 0 50 100 150 200 Tim e (min) B C 0.7 Optical Density (320nm) Optical Density (320nm) 0.7 0.6 0.5 Control 0.4 CAST 1C-2A 0.3 CAST 2C-3A 0.2 CAST 3C-4A 0.1 0.6 0.5 0.4 Control 0.3 CAST 1C-2A 0.2 CAST 2C-3A 0.1 CAST 3C-4A 0.0 0.0 0 20 40 60 0 80 20 D 60 80 E 0.7 Optical Density (320nm) 0.8 Optical Density (320nm) 40 Time (min) Time (min) 0.7 0.6 0.5 Control 0.4 CAST 1C-2A 0.3 CAST 2C-3A 0.2 CAST 3C-4A 0.1 0.0 0.6 0.5 Control 0.4 CAST 1C-2A 0.3 CAST 2C-3A 0.2 CAST 3C-4A 0.1 0.0 0 20 40 60 80 100 120 Time (min) 0 20 40 60 80 Time (min) Figure 4.3. Monitoring calpain aggregation by turbidity. (A) Aggregation of 4 µM mC105S in the presence ( ) and absence ( ) of 4 mM CaCl2. Excess EDTA (8 mM) was added to the calcium control 75 min after the initiation of the reaction. (B–E) Turbidity monitored in the presence of a 5:1 (B), 2.5:1 (C), 1.25:1 (D) and 1:1 (E) molar ratio of CAST C-A to mC105S. In each case, reactions with CAST 1C-2A ( ), 2C-3A ( ) and 3C-4A ( ) as well as a no-CAST control ( ) were performed. 60 by the sharp return to baseline absorbance following the addition of excess EDTA (final concentration 8 mM) at 80 min. After EDTA was added, there was no further increase in turbidity during the remaining 120 min of the experiment. All three CAST C-A peptides inhibited the calcium-induced aggregation of mC105S at 5:1 and 2.5:1 molar ratios of CAST to mC105S (Fig. 4.3B and C, respectively). In these experiments, the calcium control consistently reached a maximum turbidity after 15-20 min. The gradual decline in OD 320 nm thereafter can be attributed to the formation of fewer but larger aggregates resulting in a net decrease in the amount of light scattering. This is consistent with the greater fluctuations seen in the turbidity values towards the end of the experiment. It was not until a 1.25:1 molar ratio of CAST to mC105S was used that some aggregation occurred with the CAST 1C-2A and 2C-3A peptides. However, these calpastatin fragments caused a substantial delay in the onset of precipitation of ~100 and ~60 min, respectively. In addition, the amount of turbidity seen with CAST 1C-2A and 2C-3A only reached 8 % and 36 % of the control reaction, respectively. At a 1.25:1 molar ratio of peptide to enzyme, CAST 3C-4A completely inhibited the formation of aggregates (Fig. 4.3D). This trend continued when the molar ratio of CAST to mC105S was further reduced to 1:1. With CAST 1C-2A the delay in the onset of measurable aggregation fell to ~50 min and the final optical density relative to the control reaction rose to ~68 % (Fig. 4.3E). With CAST 2C-3A, the delay was determined to be ~30 min with the final OD 320 nm reaching ~80 % of the control reaction. Surprisingly, even with a 61 1:1 ratio, CAST 3C-4A inhibited aggregation as monitored by turbidity for at least 200 min. 4.5.3 CAST C-A Peptides Stabilize m-Calpain in the Presence of Ca2+ while Keeping the Active Site Clear To determine the effect of the CAST C-A peptides on the specific activity of calpain, a FRET-based assay using the substrate, (EDANS)-EPLFAER-(DABCYL), was performed (Fig. 4.4). The plot of relative fluorescence units as a function of time of reaction traced a rectangular hyperbola that was approaching a plateau at 12 min. The presence of any of the three CAST C-A peptides did not affect the rate at which calpain cleaved the FRET substrate, as the slope of the reaction curves were superimposable over the control reaction (Fig. 4.4). In addition, leupeptin quickly and completely inhibited the enzyme, further demonstrating that the C-A peptides do not block the active site or cause a conformational change that would disrupt the alignment of the catalytic triad. Therefore, we have shown that the peptides inhibit aggregation and precipitation, while keeping the active site clear to interact with target substrates as well as active-site inhibitors. Since the CAST peptides are unstructured, they are expected to be proteolytic targets of calpain. Therefore, the extent of digestion of the C-A peptides as well as their effect on autolysis of calpain was monitored by SDS-PAGE (Fig. 4.5). The no-CAST control reaction demonstrated rapid autolysis in the presence of calcium (Fig. 4.5A). 62 500 450 400 + Lep 350 Control 1c-2a RFU 300 2c-3a 250 3c-4a 200 Control + Lep 150 1c-2a + Lep 100 2c-3a + Lep 50 3c-4a + Lep 0 0 2 4 6 8 10 12 Time (min) Figure 4.4. Monitoring the effect of the CAST C-A peptides on m-calpain activity and inhibition as measured with a FRET-based assay. The activity of m-calpain (0.2 µM) in the absence of CAST ( ) was compared to its activity in the presence of a 10:1 molar ratio of CAST 1C-2A ( ), 2C-3A ( ) and 3C-4A ( ) to m-calpain and 4 mM CaCl2. Unfilled symbols represent reactions that were inhibited with the addition of 0.5 mM leupeptin (Lep) ~3 min after initiation. 63 A 0 1.5 3 5 10 15 30 E B 0 F 0 1.5 3 5 10 15 30 0 1.5 3 5 10 15 30 1.5 3 5 10 15 30 DI-IV DI-III DI-II DIV DVI 0 1.5 3 5 10 15 30 G D C 0 1.5 3 5 10 15 30 0 1.5 3 5 10 15 30 H 0 1.5 3 5 10 15 30 kDa 66 43 35 27 20 14 6.5 Figure 4.5. Autolysis of m-calpain in the presence of the CAST C-A peptides monitored by SDS-PAGE. The time points listed are in minutes. The zero time lanes for (B), (D) and (G) were run on a separate gel. The autolysis fragments of m-calpain (DI-IV, DI-III, DI-II and DVI) and bands corresponding to the C-A peptides are labelled with a star (red, green, and blue for 1C-2A, 2C-3A, and 3C-4A, respectively). Autolysis of 9 µM m-calpain was monitored in the presence of 4 mM CaCl2 and 25 µM CAST 1C-2A (B), CAST 2C-3A (C) and CAST 3C-4A (D). The effect of 0.5 mM leupeptin on autolysis was monitored in the presence of CAST 1C-2A (F), CAST 2C3A (G) and CAST 3C-4A (H). Control reactions were done in the absence of CAST C-A (A and E). 64 In particular, some protease core (domains I and II) was released within the first few minutes of autolysis and there was accumulation of DI-III and DIV as the reaction progressed. This autolysis was not noticeably inhibited by the CAST C-A peptides as judged by the decrease in the amount of 80k subunit with time and accumulation of autolysis products (Fig. 4.5B-D). The CAST C-A peptides were themselves cleaved within 1.5 min of the start of the reaction as shown by their disappearance from the gel. Given that the peptides are rapidly cleaved by calpain, the addition of an active-site calpain inhibitor (leupeptin) reduced the extent of proteolysis (Fig. 4.5F-H). There was no decrease in the staining of the 80k large calpain subunit during the 30 min incubation and no appearance of the autolysis products seen in Fig. 4.5A-D. CAST 1C-2A was the most resistant to proteolysis, as there was very little diminution of the band corresponding to the whole peptide (~10 kDa) and appearance of CAST degradation products over time (Fig. 4.5F). In the case of CAST 2C-3A, there seems to be an initial cleavage in the first minute of the assay that does not undergo further proteolysis in the remaining 60 min. This is evident since no increase in intensity of the bands corresponding to the cut peptides is shown (Fig. 4.5G). The CAST 3C-4A peptide undergoes slow but prolonged digestion, as shown by the disappearance of the band at ~14 kDa. 4.6 Discussion Autolysis coupled with aggregation and precipitation of calpain in the presence of calcium have been major obstacles to the characterization of full-length calpains. To 65 circumvent this instability, some research progress was made using the soluble protease cores. Unfortunately, the protease cores are known to be much less active than the whole enzyme and, because of the absence of domain III, they lack specificity beyond the P3 positions of substrates and inhibitors. In this study, we have used the inter-domain regions of CAST (1C-2A, 2C-3A, and 3C-4A), a calpain-specific inhibitor that is known to stabilize full-length calcium-bound calpain. This region normally resides between calpain molecules but was modeled to be long enough to span from DIV to DVI of calpain. It was our hypothesis that this would provide a link from DIV of the large subunit to DVI of the small subunit, covering hydrophobic pockets that are potential points of aggregation. In addition, if subunit dissociation actually occurs coincident with aggregation, these peptides may add stability to the interaction of the PEF domains by non-covalently cross-linking them. While maintaining the calpain stabilization of full-length CAST, the C-A peptides of CAST were used to avoid interference with small molecule inhibitors at the active site of calpain. This was shown in two ways: the C-A peptides did not affect m-calpain activity in the presence of calcium and did not interfere with the inhibition of calpain by leupeptin. Although the CAST C-A peptides prevented calpain precipitation and did not affect calpain activity, the flexible linker regions were shown to undergo proteolysis. These regions are vital to the prevention of calpain precipitation, as the individual C and A peptides are much less effective than the C-A linked peptides (Hanna et al., 66 unpublished results). Therefore, their vulnerability to proteolysis should be reduced by sequence modification. CAST 3C-4A was determined to be the best of the three peptides at preventing the precipitation of inactive m-calpain but when assayed with the active isoform, it was found to be the most readily proteolysed. This shows perhaps that the 3C-4A peptide has a stronger interaction with calpain (a lower KD) than the remaining peptides. Unfortunately, the linker region is much longer and more vulnerable to cleavage. As well as being the longest peptide, CAST 3C-4A has the most Leu (13 compared to 5 and 6 for CAST 1C-2A and 2C-3A, respectively). Leu is extremely favoured at the unprimed side of the calpain active site, where S3, S2, and S1 demonstrated a high preference for this amino acid, using a substrate library-based approach [63]. Since CAST 3C-4A was the most effective at preventing calpain precipitation, it is the obvious candidate for modification to protect it from digestion. There are several ways to reduce the vulnerability of the C-A peptides to proteolysis. The 3C-4A peptide could be further optimized by mutation of the Leu in the linker region to less favourable residues such as Pro or Ser [63]. The extended, flexible linker regions could be replaced with a rigid secondary structural element, such as a helix. This would increase the resistance of the peptides to proteolysis, as calpain prefers extended peptide regions. Since CAST 3C-4A was determined to be the best stabilizer of calpain and CAST 1C-2A was the most resistant to proteolysis, the linker region of the former could be replaced with the latter. This modified peptide could then be tested for its 67 ability to prevent precipitation by turbidity. It may be that length of the 3C-4A linker is optimal for preventing aggregation and the 1C-2A linker is too short. In addition to the improvement of CAST 3C-4A, any other combination of the C and A peptides could be tested for its effectiveness at preventing calpain precipitation while being protected from digestion. In conclusion, we have developed a way of stabilizing calpain in the presence of calcium while avoiding interference with the active site by using a portion of calpain’s natural inhibitor, CAST. The presence of the CAST peptides affects neither calpain activity nor inhibition and opens the doors for co-crystallization of full-length calpains bound to active-site inhibitors. This will aid in the structure-based design of active-site inhibitors that take advantage of added preferences provided by DIII in the unprimed positions. 68 Chapter 5 General Discussion 5.1 Is This the End of the Road for Mini-calpains? The use of the calpain protease cores has facilitated significant advances in the characterization of the active site of µ- and m-calpains. Since the full-length calpains undergo autoproteolysis and aggregation in the presence of calcium, the protease cores have been invaluable in launching the structure-based design of calpain inhibitors. The identification of the first four µI-II / inhibitor structures led to insights about structural preferences at the active site of calpain [62,64]. For example, the structure of µI-II bound to SNJ 1945 revealed that the primed-side extension of the inhibitor caused a loop displacement that exposed new sites for interaction by calpain inhibitors [62]. This was further demonstrated in the recent structure of µI-II with an α-ketoamide inhibitor that extended into and revealed novel aromatic stacking interactions with the Trp on the primed side of the cleft [61]. This compound is being used as a template for the addition of functional groups onto the inhibitor that target the primed side to increase its specificity and potency. In addition to crystallographic analysis, the protease core approach opened the doors to active site profiling with substrate and inhibitor libraries. Since the protease cores of calpain resist autoproteolysis, a peptide-library approach pioneered by Turk et al. was used in the development of a calpain substrate, PLFAER [63]. This was shown to be kinetically superior to substrates built from α- 69 spectrin or consensus sequences obtained from substrate alignments [63,65]. It also revealed sequence preferences at the primed side of the active site. A further improvement of this substrate to PLFAAR revealed a 2.3-fold higher turnover rate when tested with µI-II (see chapter 4). Sequencing of the human genome has increased the number of known calpain isoforms to 14, most of which have not yet been characterized at the protein level. Some of these isoforms are implicated in diseases such as gastric cancer (calpain 9) and type II diabetes (calpain 10), but their active-site specificity has not been determined. This is partly due to their low expression levels, making isolation from tissue difficult. The Structural Genomics Consortium (Toronto, Ontario) initiated a project to express and purify all human isoforms. Following our success with the protease cores of µ- and mcalpains they were able to produce protease cores of human calpains 1, 3, 8, 9 and 15, and determine the crystallographic structures of three: mini-calpains 1, 8 and 9 (PDB entries 2ARY, 2NQA and 1ZIV, respectively). They found that the protease cores crystallized much better with an inhibitor bound to the active site, thereby stabilizing the structure. Although the protease cores have revealed structural preferences at the active site of µ- and m-calpains, there are two main drawbacks to their use. First, they are known to be much less active than the parent enzyme. For example, µI-II is only 2-3 % as active as µ-calpain [97]. This difficulty was compounded when the activities measured with the calpain 3, 8, and 9 protease cores proved much lower than that of µI-II. For this reason, it 70 was difficult to determine inhibitor specificity even at the S2 position of mini-calpain 8. For mini-calpain 8, some of the decrease in activity can be credited to the reduced stability of the protease core. Specifically, helix α7 in DI is stable in the full-length enzyme but collapses when a Gly is present at position 203 within the helix. This hypothesis was supported by an increase in activity of mI-II when the Gly at this position was mutated to an Ala [97]. Similarly, the activity of mini-calpain 8 was increased, although to a lesser extent, by the same mutation (G203A). The second drawback associated with mini-calpains is some loss in specificity provided by DIII, one of the supportive domains of the large subunit, which affects the unprimed side of the catalytic cleft. Cuerrier et al. determined that full-length m-calpain produced preferences that were slightly different from mI-II at the unprimed positions, especially those further from the active-site cysteine (≥P3) [64]. Preliminary research on the non-classical calpain isoforms suggests that although the use of the µ- and m-calpain protease cores has been useful in the probing of substrates and inhibitors and for structure-based inhibitor design, the use of the protease cores may not be transferable to the other calpain isoforms. The different full-length calpain isoforms may demonstrate greater sequence specificity than their protease cores when compared to other cysteine proteases as well as to the other calpain isoforms. 5.2 Full-Length Calpains, the Way of the Future Given the limitations of protease cores, full-length calpains could be more useful for the development of calpain- and isoform-specific substrates and inhibitors. The 71 problems with this approach are that the full-length enzymes autolyze, aggregate, and precipitate upon calcium binding. Previous attempts at crystallizing inactive (C105S) full-length m-calpain in the presence of calcium have been difficult, due to precipitation and proposed subunit dissociation [70]. These problems were circumvented by CAST binding as demonstrated by the recent co-crystal structure of calcium-bound m-calpain and CAST4 [46]. Although CAST is known to stabilize calpain, it also inhibits it by occupying the active site. In this thesis, we have demonstrated a way of using the stabilizing property of CAST while avoiding its inhibitory potential. Although the CAST C-A peptides inhibited aggregation of inactive calpain, they are readily cleaved by the active enzyme. Their unstructured, extended conformation renders them susceptible to proteolysis. This vulnerability may be reduced by the mutation of residues that are known to be favoured in the active site of calpain. The linkers could be scanned for sequences that match the optimal PLFAER and PLFAAR cleavage sequences at two or more sites, especially ones that have Leu, Ile and Val in the S2 subsite. For example, one of the prominent autoproteolysis sites in calpain between domains III and IV is RVFSEK [114]. This matches at two positions with a further three conservative substitutions. It would be relatively easy to spoil this cleavage site by mutation of VF to hydrophilic residues. The addition of Pro in the peptides may also provide protection from proteolysis as cleavage is restricted to the C-terminal side of that amino acid. The insertion of secondary structural elements that are resistant to calpainmediated cleavage would also decrease digestion of the CAST C-A peptides. 72 The applications of this method of calpain stabilization are numerous, including substrate and inhibitor specificity determination at the unprimed side of calpain where DIII helps form the S3 and S4 and outer unprimed sub-sites. So long as the CAST C-A peptides are protected from calpain digestion; the library-based approaches described previously can be extended to the stabilized full-length calpain. Following the identification of an optimal substrate sequence, the method developed by Polster et al. with PLFAER could be used to produce cell-permeable biomarkers of calpain overactivation in cells [106]. In addition, structure-based design of calpain inhibitors with stabilized full-length calpain that target both the active site as well as allosteric positions can now be pursued. Compounds that have been shown to extend past subsites covered by the protease core alone, such as WR13 (R,R) and WR18 (S,S) would be valuable targets for the cocrystallization with the stabilized whole enzyme [64]. These inhibitors have an Nterminal Trp where no density appeared in the inhibitor-bound µI-II structure. This can be attributed to the lack of DIII, which would provide a site of interaction to hold the residue in place in the whole enzyme. In addition, the N-terminal region of SNJ 1945 was crystallized in two conformations, suggesting flexibility that may be restricted by DIII in the full-length enzyme [62]. These methodologies can also be extended to other calpain isoforms if they can be produced as active enzymes. Unfortunately, these isoforms are extremely difficult to produce and purify. Calpain 3 is a case in point where the insertion sequences seem to 73 predispose the enzyme to autoproteolysis [115]. Our laboratory tried to express just the protease core of calpain 3 (containing insertion sequence 1) but it was subject to inexorable intramolecular autoproteolysis even in the absence of exogenous calcium [116]. Because of these problems, it may be useful to produce pseudo-full-length calpains. That is, the protease cores of these isoforms could potentially be inserted into DIII-DVI of m-calpain. In this way, m-calpain would act as a surrogate stabilizer for profiling and structure-based inhibitor design of the non-classical calpain isoforms. This may lead to the development of isoform-specific calpain substrates and inhibitors that will help clarify their cellular roles. 5.3 Applications of Allosteric Inhibitor Design Much of this thesis has focused on the determination of the active-site specificity of calpain isoforms. To date, the search for calpain-specific inhibitors targeting the active site has been relatively unsuccessful, mostly due to cross-reactivity with other cysteine proteases. The stabilization of full-length calpain by the CAST peptides proposed in chapter 4 of this thesis may provide the necessary advantage to begin inhibitor screening projects. In particular, the development of inhibitors that inactivate calpain allosterically would be of significant pharmacological interest as they would diversify the available compounds and be less likely to cross-react with other proteases. Examples of inhibitors that bind distal to the active site include the novel caspase1, -3 and -7 inhibitors revealed by disulfide trapping, a fragment-based drug discovery approach [117,118]. These compounds interact with a deep cavity at the dimerization 74 interface 15 Å away from the active site and lock the enzymes in an inactive zymogenlike conformation. Similarly, recent structural data have revealed an allosteric inhibitor of the caspase 2 isoform that covalently binds to the dimer interface resulting in slight structural changes that promote a zymogen-like conformation, including a displacement of 1 Å of the active-site cysteine [119]. The development of calpain-specific allosteric inhibitors has been minimal to date. Two compounds, PD150606 and PD151746, are examples of such inhibitors that target the calcium-binding PEF domains of calpain [120]. PD150606 was determined to be an allosteric inhibitor as it did not block or compete with substrate binding at the active site [120]. X-ray crystallographic analysis demonstrated that PD150606 interacts with the same hydrophobic patch in DVI of calpain as the CAST C peptide [69]. Both inhibitors were specific for µ- and m-calpains over other proteases [120]. It is important to point out that in this thesis neither the C peptide alone nor the CA peptides have been shown to alter calpain activity. Two separate groups have published contradictory evidence that interaction at the hydrophobic pocket occupied by the CAST C peptide affects the activity of calpain. As mentioned above, Wang et al. have proposed that PD150606 inhibits calpain through interaction at this site [120]. In addition, Tompa et al. have postulated that the CAST C peptide acts as an activator of calpain [121]. It is difficult to resolve these hypotheses as they were neither evident in our results nor have they been reported elsewhere. With respect to the PD150606 inhibitor, it was crystallized with DVI of calpain alone. In order to verify that the inhibitor does not act on another 75 allosteric site within calpain, it should be crystallized with the full-length enzyme. In addition, a FRET assay can be done with the protease core of calpain to determine whether it can inhibit at a site within DI and DII. The isoform-specific caspase allosteric inhibitors described above demonstrate the importance of developing compounds that have an indirect mechanism of action through structural rearrangements. With the limited specificity achieved thus far with active-site inhibitors of calpain, probing of allosteric compounds may reduce cross-reactivity with other cysteine proteases. Since calpains are activated by calcium binding to the protease core domains DI and DII as well as the PEF domains DIV and DVI, targeting these sites in the calcium-free form might prevent calcium binding and therefore calpain activation. With the recent stabilization of full-length calcium-bound calpain by CAST C-A, a screening approach can be attempted to isolate compounds and/or proteins that interact with calpain allosterically. This may lead to the development of calpain- and isoformspecific allosteric inhibitors for pharmacological drug design as well as possible calpain binding partners in an attempt to clarify their cellular roles. 5.4 Summary and Conclusions 1. The mini-calpains 3, 8 and 9 were determined to have much lower activities than µI-II when using inactive m-calpain (mC105S) as a substrate. In the same experiment, mini-calpain 15 could not digest mC105S, highlighting the inactivity of this isoform. 76 2. The activity of mini-calpain 8 was improved by the mutation of Gly203 to an Ala, which is proposed to stabilize a helix in the protease core and prevent Trp106 from interrupting the active site. 3. Mini-calpain 8 was shown to have similar preferences as µI-II and mI-II for small aliphatic amino acids at the P2 position. 4. The site of calpain-mediated cleavage of the FRET substrate (EDANS)-EPLFMER(DABCYL) between the F and M residues was confirmed using mass spectrometry. 5. The FRET substrate, (EDANS)-EPLFAERK-(DABCYL) was further optimized to (EDANS)-EPLFAARK-(DABCYL), which was turned-over by µ-calpain 2.3-fold more rapidly. 6. Peptides engineered from the endogenous inhibitor of calpain, CAST, were shown to inhibit the aggregation of full-length m-calpain in the presence of calcium. The peptides were cloned from the C sub-domain of one CAST domain to the A subdomain of the next, producing three peptides (CAST 1C-2A, 2C-3A and 3C-4A). CAST 3C-4A was revealed through turbidity analysis to inhibit calpain aggregation at a 1:1 molar ratio of peptide to enzyme. 7. The CAST C-A peptides did not effect either the activity or autolysis of calpain but were shown to be cleaved by calpain in the absence of an active-site inhibitor. 77 References 1. Ringer,S. (1883). A further Contribution regarding the influence of the different Constituents of the Blood on the Contraction of the Heart. J. Physiol 4, 29-42. 2. Verkhratsky,A. and Toescu, E. C. (2003). Endoplasmic reticulum Ca(2+) homeostasis and neuronal death. J. Cell Mol. Med. 7, 351-361. 3. Paschen,W. and Mengesdorf, T. (2005). Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium 38, 409-415. 4. Leist,M. and Nicotera, P. (1998). Apoptosis versus necrosis: the shape of neuronal cell death. Results Probl. Cell Differ. 24, 105-135. 5. Berliocchi,L., Bano, D., and Nicotera, P. (2005). Ca2+ signals and death programmes in neurons. Philos. Trans. R. Soc. Lond B Biol. Sci. 360, 2255-2258. 6. Brehm,P. and Eckert, R. (1978). Calcium entry leads to inactivation of calcium channel in Paramecium. Science 202, 1203-1206. 7. Marban,E. and Tsien, R. W. (1982). Enhancement of calcium current during digitalis inotropy in mammalian heart: positive feed-back regulation by intracellular calcium? J. Physiol 329, 589-614. 8. Spitzer,N.C. (2008). Calcium: first messenger. Nat. Neurosci. 11, 243-244. 9. Carafoli,E. (2005). Calcium--a universal carrier of biological signals. Delivered on 3 July 2003 at the Special FEBS Meeting in Brussels. FEBS J. 272, 1073-1089. 78 10. Murachi,T., Tanaka, K., Hatanaka, M., and Murakami, T. (1980). Intracellular Ca2+dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin). Adv. Enzyme Regul. 19, 407-424. 11. Barret,A.J., Rawlings, N. D., and Woessner, J. F. (1998). Hanbook of Proteolytic Enzymes. 12. GUROFF,G. (1964). A neutral, calciu-activated proteinase from the soluble fraction of rat brain. J. Biol. Chem. 239, 149-155. 13. Dayton,W.R., Goll, D. E., Zeece, M. G., Robson, R. M., and Reville, W. J. (1976). A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Purification from porcine muscle. Biochemistry 15, 2150-2158. 14. Goll,D.E., Thompson, V. F., Li, H., Wei, W., and Cong, J. (2003). The calpain system. Physiol Rev. 83, 731-801. 15. Glading,A., Lauffenburger, D. A., and Wells, A. (2002). Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 12, 46-54. 16. Janossy,J., Ubezio, P., Apati, A., Magocsi, M., Tompa, P., and Friedrich, P. (2004). Calpain as a multi-site regulator of cell cycle. Biochem. Pharmacol. 67, 15131521. 17. Neumar,R.W., Xu, Y. A., Gada, H., Guttmann, R. P., and Siman, R. (2003). Cross-talk between calpain and caspase proteolytic systems during neuronal apoptosis. J. Biol. Chem. 278, 14162-14167. 18. Dutt,P., Croall, D. E., Arthur, J. S., Veyra, T. D., Williams, K., Elce, J. S., and Greer, P. A. (2006). m-Calpain is required for preimplantation embryonic development in mice. BMC. Dev. Biol. 6, 319. Zimmerman,U.J., Boring, L., Pak, J. H., Mukerjee, N., and Wang, K. K. (2000). The calpain small subunit gene is essential: its inactivation results in embryonic lethality. IUBMB. Life 50, 63-68. 20. Arthur,J.S., Elce, J. S., Hegadorn, C., Williams, K., and Greer, P. A. (2000). Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol. Cell Biol. 20, 4474-4481. 21. Grammer,M., Kuchay, S., Chishti, A., and Baudry, M. (2005). Lack of phenotype for LTP and fear conditioning learning in calpain 1 knock-out mice. Neurobiol. Learn. Mem. 84, 222-227. 22. Azam,M., Andrabi, S. S., Sahr, K. E., Kamath, L., Kuliopulos, A., and Chishti, A. H. (2001). Disruption of the mouse mu-calpain gene reveals an essential role in platelet function. Mol. Cell Biol. 21, 2213-2220. 23. Badugu,R., Garcia, M., Bondada, V., Joshi, A., and Geddes, J. W. (2008). N terminus of calpain 1 is a mitochondrial targeting sequence. J. Biol. Chem. 283, 3409-3417. 24. Garcia,M., Bondada, V., and Geddes, J. W. (2005). Mitochondrial localization of mucalpain. Biochem. Biophys. Res. Commun. 338, 1241-1247. 25. Kramerova,I., Kudryashova, E., Tidball, J. G., and Spencer, M. J. (2004). Null mutation of calpain 3 (p94) in mice causes abnormal sarcomere formation in vivo and in vitro. Hum. Mol. Genet. 13, 1373-1388. 26. Bevers,M.B. and Neumar, R. W. (2008). Mechanistic role of calpains in postischemic neurodegeneration. J. Cereb. Blood Flow Metab 28, 655-673. 79 27. Saito,K., Elce, J. S., Hamos, J. E., and Nixon, R. A. (1993). Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc. Natl. Acad. Sci. U. S. A 90, 2628-2632. 28. Nixon,R.A., Saito, K. I., Grynspan, F., Griffin, W. R., Katayama, S., Honda, T., Mohan, P. S., Shea, T. B., and Beermann, M. (1994). Calcium-activated neutral proteinase (calpain) system in aging and Alzheimer's disease. Ann. N. Y. Acad. Sci. 747, 77-91. 29. Crocker,S.J., Smith, P. D., Jackson-Lewis, V., Lamba, W. R., Hayley, S. P., Grimm, E., Callaghan, S. M., Slack, R. S., Melloni, E., Przedborski, S., Robertson, G. S., Anisman, H., Merali, Z., and Park, D. S. (2003). Inhibition of calpains prevents neuronal and behavioral deficits in an MPTP mouse model of Parkinson's disease. J. Neurosci. 23, 4081-4091. 30. Gafni,J., Hermel, E., Young, J. E., Wellington, C. L., Hayden, M. R., and Ellerby, L. M. (2004). Inhibition of calpain cleavage of huntingtin reduces toxicity: accumulation of calpain/caspase fragments in the nucleus. J. Biol. Chem. 279, 20211-20220. 31. Vanderklish,P.W. and Bahr, B. A. (2000). The pathogenic activation of calpain: a marker and mediator of cellular toxicity and disease states. Int. J. Exp. Pathol. 81, 323339. 32. Camins,A., Verdaguer, E., Folch, J., and Pallas, M. (2006). Involvement of calpain activation in neurodegenerative processes. CNS. Drug Rev. 12, 135-148. 33. Lee,M.S., Kwon, Y. T., Li, M., Peng, J., Friedlander, R. M., and Tsai, L. H. (2000). Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405, 360-364. 34. Patrick,G.N., Zukerberg, L., Nikolic, M., de la, Monte S., Dikkes, P., and Tsai, L. H. (1999). Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402, 615-622. 35. Badugu,R., Garcia, M., Bondada, V., Joshi, A., and Geddes, J. W. (2008). N terminus of calpain 1 is a mitochondrial targeting sequence. J. Biol. Chem. 283, 3409-3417. 36. Berti,P.J. and Storer, A. C. (1995). Alignment/phylogeny of the papain superfamily of cysteine proteases. J. Mol. Biol. 246, 273-283. 37. Hosfield,C.M., Ye, Q., Arthur, J. S., Hegadorn, C., Croall, D. E., Elce, J. S., and Jia, Z. (1999). Crystallization and X-ray crystallographic analysis of m-calpain, a Ca2+dependent protease. Acta Crystallogr. D. Biol. Crystallogr. 55, 1484-1486. 38. Sutton,R.B., Davletov, B. A., Berghuis, A. M., Sudhof, T. C., and Sprang, S. R. (1995). Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipidbinding fold. Cell 80, 929-938. 39. Blanchard,H., Grochulski, P., Li, Y., Arthur, J. S., Davies, P. L., Elce, J. S., and Cygler, M. (1997). Structure of a calpain Ca(2+)-binding domain reveals a novel EFhand and Ca(2+)-induced conformational changes. Nat. Struct. Biol. 4, 532-538. 40. Lin,G.D., Chattopadhyay, D., Maki, M., Wang, K. K., Carson, M., Jin, L., Yuen, P. W., Takano, E., Hatanaka, M., DeLucas, L. J., and Narayana, S. V. (1997). Crystal structure of calcium bound domain VI of calpain at 1.9 A resolution and its role in enzyme assembly, regulation, and inhibitor binding. Nat. Struct. Biol. 4, 539547. 80 41. Ravulapalli,R., Diaz, B. G., Campbell, R. L., and Davies, P. L. (2005). Homodimerization of calpain 3 penta-EF-hand domain. Biochem. J. 388, 585591. 42. Strobl,S., Fernandez-Catalan, C., Braun, M., Huber, R., Masumoto, H., Nakagawa, K., Irie, A., Sorimachi, H., Bourenkow, G., Bartunik, H., Suzuki, K., and Bode, W. (2000). The crystal structure of calcium-free human m-calpain suggests an electrostatic switch mechanism for activation by calcium. Proc. Natl. Acad. Sci. U. S. A 97, 588-592. 43. Moldoveanu,T., Campbell, R. L., Cuerrier, D., and Davies, P. L. (2004). Crystal structures of calpain-E64 and -leupeptin inhibitor complexes reveal mobile loops gating the active site. J. Mol. Biol. 343, 1313-1326. 44. Moldoveanu,T., Hosfield, C. M., Lim, D., Elce, J. S., Jia, Z., and Davies, P. L. (2002). A Ca(2+) switch aligns the active site of calpain. Cell 108, 649-660. 45. Tompa,P., Emori, Y., Sorimachi, H., Suzuki, K., and Friedrich, P. (2001). Domain III of calpain is a ca2+-regulated phospholipid-binding domain. Biochem. Biophys. Res. Commun. 280, 1333-1339. 46. Hanna,R., Campbell, R. L., and Davies, P. L (2008). Calcium-bound structure of calpain and its mechanism of inhibition by the endogenous inhibitor, calpastatin. Nature. In Press 47. Blanchard,H., Grochulski, P., Li, Y., Arthur, J. S., Davies, P. L., Elce, J. S., and Cygler, M. (1997). Structure of a calpain Ca(2+)-binding domain reveals a novel EFhand and Ca(2+)-induced conformational changes. Nat. Struct. Biol. 4, 532-538. 48. Lin,G.D., Chattopadhyay, D., Maki, M., Wang, K. K., Carson, M., Jin, L., Yuen, P. W., Takano, E., Hatanaka, M., DeLucas, L. J., and Narayana, S. V. (1997). Crystal structure of calcium bound domain VI of calpain at 1.9 A resolution and its role in enzyme assembly, regulation, and inhibitor binding. Nat. Struct. Biol. 4, 539547. 49. Suzuki,K., Hata, S., Kawabata, Y., and Sorimachi, H. (2004). Structure, activation, and biology of calpain. Diabetes 53 Suppl 1, S12-S18. 50. Garcia Diaz,B.E., Gauthier, S., and Davies, P. L. (2006). Ca2+ dependency of calpain 3 (p94) activation. Biochemistry 45, 3714-3722. 51. Sorimachi,H., Toyama-Sorimachi, N., Saido, T. C., Kawasaki, H., Sugita, H., Miyasaka, M., Arahata, K., Ishiura, S., and Suzuki, K. (1993). Muscle-specific calpain, p94, is degraded by autolysis immediately after translation, resulting in disappearance from muscle. J. Biol. Chem. 268, 10593-10605. 52. Sorimachi,H., Kinbara, K., Kimura, S., Takahashi, M., Ishiura, S., Sasagawa, N., Sorimachi, N., Shimada, H., Tagawa, K., Maruyama, K., and . (1995). Musclespecific calpain, p94, responsible for limb girdle muscular dystrophy type 2A, associates with connectin through IS2, a p94-specific sequence. J. Biol. Chem. 270, 31158-31162. 53. Sorimachi,H., Imajoh-Ohmi, S., Emori, Y., Kawasaki, H., Ohno, S., Minami, Y., and Suzuki, K. (1989). Molecular cloning of a novel mammalian calcium-dependent protease distinct from both m- and mu-types. Specific expression of the mRNA in skeletal muscle. J. Biol. Chem. 264, 20106-20111. 54. Richard,I., Broux, O., Allamand, V., Fougerousse, F., Chiannilkulchai, N., Bourg, N., Brenguier, L., Devaud, C., Pasturaud, P., Roudaut, C., and . (1995). Mutations in 81 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. 66. 67. 68. 69. the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell 81, 27-40. Sorimachi, H. and Beckmann, J. S. 2002. Defects of non-lysosomal proteolysis: calpain 3 deficiency.In: Structural and molecular basis of skeletal muscle diseases, edited by Karpati, G. Basel, Switzerland Neuropath Press, 148-153. Hata,S., Koyama, S., Kawahara, H., Doi, N., Maeda, T., Toyama-Sorimachi, N., Abe, K., Suzuki, K., and Sorimachi, H. (2005). Stomach-specific Calpain, nCL-2. Localizes in Mucus Cells and Proteolyzes the Beta-Subunit of Coatomer Complex, Beta-COP. The Journal of Biological Chemistry 281, 11214-11224. Yoshikawa,Y., Mukai, H., Hino, F., Asada, K., and Kato, I. (2000). Isolation of two novel genes, down-regulated in gastric cancer. Jpn. J. Cancer Res. 91, 459-463. Liu,K., Li, L., and Cohen, S. N. (2000). Antisense RNA-mediated deficiency of the calpain protease, nCL-4, in NIH3T3 cells is associated with neoplastic transformation and tumorigenesis. J. Biol. Chem. 275, 31093-31098. Dear,T.N., Moller, A., and Boehm, T. (1999). CAPN11: A calpain with high mRNA levels in testis and located on chromosome 6. Genomics 59, 243-247. Dear,T.N., Meier, N. T., Hunn, M., and Boehm, T. (2000). Gene structure, chromosomal localization, and expression pattern of Capn12, a new member of the calpain large subunit gene family. Genomics 68, 152-160. Qian,J., Cuerrier, D., Davies, P. L., Li, Z., Powers, J. C., and Campbell, R. L. (2008). Cocrystal Structures of Primed Side-Extending alpha-Ketoamide Inhibitors Reveal Novel Calpain-Inhibitor Aromatic Interactions. J. Med. Chem. Cuerrier,D., Moldoveanu, T., Inoue, J., Davies, P. L., and Campbell, R. L. (2006). Calpain inhibition by alpha-ketoamide and cyclic hemiacetal inhibitors revealed by X-ray crystallography. Biochemistry 45, 7446-7452. Cuerrier,D., Moldoveanu, T., and Davies, P. L. (2005). Determination of peptide substrate specificity for mu-calpain by a peptide library-based approach: the importance of primed side interactions. J. Biol. Chem. 280, 40632-40641. Cuerrier,D., Moldoveanu, T., Campbell, R. L., Kelly, J., Yoruk, B., Verhelst, S. H., Greenbaum, D., Bogyo, M., and Davies, P. L. (2007). Development of calpainspecific inactivators by screening of positional scanning epoxide libraries. J. Biol. Chem. 282, 9600-9611. Turk,B.E., Huang, L. L., Piro, E. T., and Cantley, L. C. (2001). Determination of protease cleavage site motifs using mixture-based oriented peptide libraries. Nat. Biotechnol. 19, 661-667. Schechter,I. and Berger, A. (1967). On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 27, 157-162. Li,H., Thompson, V. F., and Goll, D. E. (2004). Effects of autolysis on properties of muand m-calpain. Biochim. Biophys. Acta 1691, 91-103. Zimmerman,U.J. and Schlaepfer, W. W. (1991). Two-stage autolysis of the catalytic subunit initiates activation of calpain I. Biochim. Biophys. Acta 1078, 192-198. Todd,B., Moore, D., Deivanayagam, C. C., Lin, G. D., Chattopadhyay, D., Maki, M., Wang, K. K., and Narayana, S. V. (2003). A structural model for the inhibition of calpain by calpastatin: crystal structures of the native domain VI of calpain and its complexes with calpastatin peptide and a small molecule inhibitor. J. Mol. Biol. 328, 131-146. 82 70. Pal,G.P., Elce, J. S., and Jia, Z. (2001). Dissociation and aggregation of calpain in the presence of calcium. J. Biol. Chem. 276, 47233-47238. 71. Okitani,A., Goll, D. E., Stromer, M. H., and Robson, R. M. (1976). Intracellular inhibitor of Ca2+-activated protease involved in myofibrillar protein turnover. Federation Proceedings 35, 174672. Murachi,T. (1989). Intracellular regulatory system involving calpain and calpastatin. Biochem. Int. 18, 263-294. 73. Emori,Y., Kawasaki, H., Imajoh, S., Imahori, K., and Suzuki, K. (1987). Endogenous inhibitor for calcium-dependent cysteine protease contains four internal repeats that could be responsible for its multiple reactive sites. Proc. Natl. Acad. Sci. U. S. A 84, 3590-3594. 74. Emori,Y., Kawasaki, H., Imajoh, S., Minami, Y., and Suzuki, K. (1988). All four repeating domains of the endogenous inhibitor for calcium-dependent protease independently retain inhibitory activity. Expression of the cDNA fragments in Escherichia coli. J. Biol. Chem. 263, 2364-2370. 75. Maki,M., Takano, E., Mori, H., Sato, A., Murachi, T., and Hatanaka, M. (1987). All four internally repetitive domains of pig calpastatin possess inhibitory activities against calpains I and II. FEBS Lett. 223, 174-180. 76. Takano, E., Masatoshi, M., and Yuen, P. 1999. Structure of calpastatin and its inhibitory control of calpain.In: Calpain: pharmacology and toxicology of calciumdependent protease, edited by Wang, K. K. W and Yuen, P. W. Philadelphia 2550. 77. Bokor,M., Csizmok, V., Kovacs, D., Banki, P., Friedrich, P., Tompa, P., and Tompa, K. (2005). NMR relaxation studies on the hydrate layer of intrinsically unstructured proteins. Biophys. J. 88, 2030-2037. 78. Imajoh,S., Kawasaki, H., Emori, Y., and Suzuki, K. (1987). Calcium-activated neutral protease inhibitor from rabbit erythrocytes lacks the N-terminal region of the liver inhibitor but retains three inhibitory units. Biochem. Biophys. Res. Commun. 146, 630-637. 79. Wang,L.F., Wei, S. G., Miao, S. Y., Liu, Q. Y., and Koide, S. S. (1994). Calpastatin gene in human testis. Biochem. Mol. Biol. Int. 33, 245-251. 80. Cong,M., Thompson, V. F., Goll, D. E., and Antin, P. B. (1998). The bovine calpastatin gene promoter and a new N-terminal region of the protein are targets for cAMPdependent protein kinase activity. J. Biol. Chem. 273, 660-666. 81. Lee,W.J., Ma, H., Takano, E., Yang, H. Q., Hatanaka, M., and Maki, M. (1992). Molecular diversity in amino-terminal domains of human calpastatin by exon skipping. J. Biol. Chem. 267, 8437-8442. 82. Takano,J., Kawamura, T., Murase, M., Hitomi, K., and Maki, M. (1999). Structure of mouse calpastatin isoforms: implications of species-common and species-specific alternative splicing. Biochem. Biophys. Res. Commun. 260, 339-345. 83. Maki,M., Takano, E., Osawa, T., Ooi, T., Murachi, T., and Hatanaka, M. (1988). Analysis of structure-function relationship of pig calpastatin by expression of mutated cDNAs in Escherichia coli. J. Biol. Chem. 263, 10254-10261. 84. Uemori,T., Shimojo, T., Asada, K., Asano, T., Kimizuka, F., Kato, I., Maki, M., Hatanaka, M., Murachi, T., Hanzawa, H., and . (1990). Characterization of a 83 85. 86. 87. 88. 89. 90. 91. 92. 93. 94. 95. 96. 97. 98. functional domain of human calpastatin. Biochem. Biophys. Res. Commun. 166, 1485-1493. Yang,H.Q., Ma, H., Takano, E., Hatanaka, M., and Maki, M. (1994). Analysis of calcium-dependent interaction between amino-terminal conserved region of calpastatin functional domain and calmodulin-like domain of mu-calpain large subunit. J. Biol. Chem. 269, 18977-18984. Takano,E., Ma, H., Yang, H. Q., Maki, M., and Hatanaka, M. (1995). Preference of calcium-dependent interactions between calmodulin-like domains of calpain and calpastatin subdomains. FEBS Lett. 362, 93-97. Hanna,R.A., Garcia-Diaz, B. E., and Davies, P. L. (2007). Calpastatin simultaneously binds four calpains with different kinetic constants. FEBS Lett. 581, 2894-2898. Kawasaki,H., Emori, Y., Imajoh-Ohmi, S., Minami, Y., and Suzuki, K. (1989). Identification and characterization of inhibitory sequences in four repeating domains of the endogenous inhibitor for calcium-dependent protease. J. Biochem. 106, 274-281. Maki,M., Takano, E., Osawa, T., Ooi, T., Murachi, T., and Hatanaka, M. (1988). Analysis of structure-function relationship of pig calpastatin by expression of mutated cDNAs in Escherichia coli. J. Biol. Chem. 263, 10254-10261. Croall,D.E. and McGrody, K. S. (1994). Domain structure of calpain: mapping the binding site for calpastatin. Biochemistry 33, 13223-13230. Sorimachi,H. and Suzuki, K. (2001). The structure of calpain. J. Biochem. 129, 653-664. Sorimachi,H., Ishiura, S., and Suzuki, K. (1993). A novel tissue-specific calpain species expressed predominantly in the stomach comprises two alternative splicing products with and without Ca(2+)-binding domain. J. Biol. Chem. 268, 1947619482. Lee,H.J., Sorimachi, H., Jeong, S. Y., Ishiura, S., and Suzuki, K. (1998). Molecular cloning and characterization of a novel tissue-specific calpain predominantly expressed in the digestive tract. Biol. Chem. 379, 175-183. Lee,H.J., Tomioka, S., Kinbara, K., Masumoto, H., Jeong, S. Y., Sorimachi, H., Ishiura, S., and Suzuki, K. (1999). Characterization of a human digestive tract-specific calpain, nCL-4, expressed in the baculovirus system. Arch. Biochem. Biophys. 362, 22-31. Kamei,M., Webb, G. C., Heydon, K., Hendry, I. A., Young, I. G., and Campbell, H. D. (2000). Solh, the mouse homologue of the Drosophila melanogaster small optic lobes gene: organization, chromosomal mapping, and localization of gene product to the olfactory bulb. Genomics 64, 82-89. Greenbaum,D., Medzihradszky, K. F., Burlingame, A., and Bogyo, M. (2000). Epoxide electrophiles as activity-dependent cysteine protease profiling and discovery tools. Chem. Biol. 7, 569-581. Moldoveanu,T., Hosfield, C. M., Lim, D., Jia, Z., and Davies, P. L. (2003). Calpain silencing by a reversible intrinsic mechanism. Nat. Struct. Biol. 10, 371-378. Cuerrier,D., Moldoveanu, T., and Davies, P. L. (2005). Determination of peptide substrate specificity for mu-calpain by a peptide library-based approach: the importance of primed side interactions. J. Biol. Chem. 280, 40632-40641. 84 99. Croce,K., Flaumenhaft, R., Rivers, M., Furie, B., Furie, B. C., Herman, I. M., and Potter, D. A. (1999). Inhibition of calpain blocks platelet secretion, aggregation, and spreading. J. Biol. Chem. 274, 36321-36327. 100. Tompa,P., Buzder-Lantos, P., Tantos, A., Farkas, A., Szilagyi, A., Banoczi, Z., Hudecz, F., and Friedrich, P. (2004). On the sequential determinants of calpain cleavage. J. Biol. Chem. 279, 20775-20785. 101. Johnson,G.V., Jope, R. S., and Binder, L. I. (1989). Proteolysis of tau by calpain. Biochem. Biophys. Res. Commun. 163, 1505-1511. 102. Hayashi,M., Suzuki, H., Kawashima, S., Saido, T. C., and Inomata, M. (1999). The behavior of calpain-generated N- and C-terminal fragments of talin in integrinmediated signaling pathways. Arch. Biochem. Biophys. 371, 133-141. 103. Du,X., Saido, T. C., Tsubuki, S., Indig, F. E., Williams, M. J., and Ginsberg, M. H. (1995). Calpain cleavage of the cytoplasmic domain of the integrin beta 3 subunit. J. Biol. Chem. 270, 26146-26151. 104. Sasaki,T., Kikuchi, T., Yumoto, N., Yoshimura, N., and Murachi, T. (1984). Comparative specificity and kinetic studies on porcine calpain I and calpain II with naturally occurring peptides and synthetic fluorogenic substrates. J. Biol. Chem. 259, 12489-12494. 105. Sasaki,T., Kikuchi, T., Fukui, I., and Murachi, T. (1986). Inactivation of calpain I and calpain II by specificity-oriented tripeptidyl chloromethyl ketones. J. Biochem. 99, 173-179. 106. Polster,B.M., Arze, R., Lyttle, M. H., Nicholls, D. G., and Hudson, D. (2007). Solid Phase Synthesis of Dual Labeled Peptides: Development of Cell Permeable Calpain Specific Substrates. International Journal of Peptide Research and Therapeutics 13, 83-91. 107. Liu,Y., Kati, W, Chen, C, Tripathi, R, Molla, A, and Kohlbrenner, W (1999). Use of a Fluorescence Plate Reader for Measuring Kinetic Parameters with Inner Filter Effect Correction. Analytical Biochemistry 267, 331-335. 108. Davis,T.L., Walker, J. R., Finerty, P. J., Jr., Mackenzie, F., Newman, E. M., and DhePaganon, S. (2007). The crystal structures of human calpains 1 and 9 imply diverse mechanisms of action and auto-inhibition. J. Mol. Biol. 366, 216-229. 109. Saez,M.E., Ramirez-Lorca, R., Moron, F. J., and Ruiz, A. (2006). The therapeutic potential of the calpain family: new aspects. Drug Discov. Today 11, 917-923. 110. Cottin,P., Vidalenc, P. L., and Ducastaing, A. (1981). Ca2+-dependent association between a Ca2+-activated neutral proteinase (CaANP) and its specific inhibitor. FEBS Lett. 136, 221-224. 111. Mucsi,Z., Hudecz, F., Hollosi, M., Tompa, P., and Friedrich, P. (2003). Binding-induced folding transitions in calpastatin subdomains A and C. Protein Sci. 12, 23272336. 112. Yoshizawa,T., Sorimachi, H., Tomioka, S., Ishiura, S., and Suzuki, K. (1995). Calpain dissociates into subunits in the presence of calcium ions. Biochem. Biophys. Res. Commun. 208, 376-383. 113. Elce,J.S., Hegadorn, C., Gauthier, S., Vince, J. W., and Davies, P. L. (1995). Recombinant calpain II: improved expression systems and production of a C105A active-site mutant for crystallography. Protein Eng 8, 843-848. 85 114. Crawford,C., Brown, N. R., and Willis, A. C. (1993). Studies of the active site of mcalpain and the interaction with calpastatin. Biochem. J. 296 ( Pt 1), 135-142. 115. Sorimachi,H., Toyama-Sorimachi, N., Saido, T. C., Kawasaki, H., Sugita, H., Miyasaka, M., Arahata, K., Ishiura, S., and Suzuki, K. (1993). Muscle-specific calpain, p94, is degraded by autolysis immediately after translation, resulting in disappearance from muscle. J. Biol. Chem. 268, 10593-10605. 116. Garcia Diaz,B.E., Gauthier, S., and Davies, P. L. (2006). Ca2+ dependency of calpain 3 (p94) activation. Biochemistry 45, 3714-3722. 117. Hardy,J.A., Lam, J., Nguyen, J. T., O'Brien, T., and Wells, J. A. (2004). Discovery of an allosteric site in the caspases. Proc. Natl. Acad. Sci. U. S. A 101, 12461-12466. 118. Scheer,J.M., Romanowski, M. J., and Wells, J. A. (2006). A common allosteric site and mechanism in caspases. Proc. Natl. Acad. Sci. U. S. A 103, 7595-7600. 119. Schweizer,A., Roschitzki-Voser, H., Amstutz, P., Briand, C., Gulotti-Georgieva, M., Prenosil, E., Binz, H. K., Capitani, G., Baici, A., Pluckthun, A., and Grutter, M. G. (2007). Inhibition of caspase-2 by a designed ankyrin repeat protein: specificity, structure, and inhibition mechanism. Structure. 15, 625-636. 120. Wang,K.K., Nath, R., Posner, A., Raser, K. J., Buroker-Kilgore, M., Hajimohammadreza, I., Probert, A. W., Jr., Marcoux, F. W., Ye, Q., Takano, E., Hatanaka, M., Maki, M., Caner, H., Collins, J. L., Fergus, A., Lee, K. S., Lunney, E. A., Hays, S. J., and Yuen, P. (1996). An alpha-mercaptoacrylic acid derivative is a selective nonpeptide cell-permeable calpain inhibitor and is neuroprotective. Proc. Natl. Acad. Sci. U. S. A 93, 6687-6692. 121. Tompa,P., Mucsi, Z., Orosz, G., and Friedrich, P. (2002). Calpastatin subdomains A and C are activators of calpain. J. Biol. Chem. 277, 9022-9026. 86