* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download presentation source
Asymmetric induction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Aromaticity wikipedia , lookup
Polythiophene wikipedia , lookup
George S. Hammond wikipedia , lookup
Hydroformylation wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Ene reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
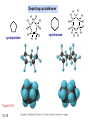
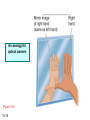
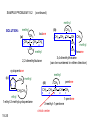
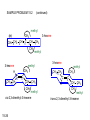
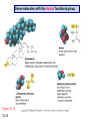
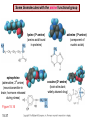
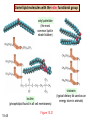
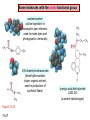
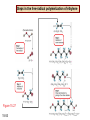
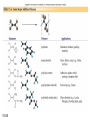
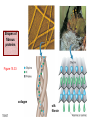
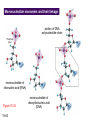
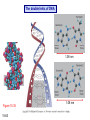
Chapter 15 Organic Compounds and the Atomic Properties of Carbon 15-1 Organic Compounds and the Atomic Properties of Carbon 15.1 The special nature of carbon and the characteristics of organic molecules 15.2 The structures and classes of hydrocarbons 15.3 Some important classes of organic reactions 15.4 Properties and reactivities of common functional groups 15.5 The monomer-polymer theme I: Synthetic macromolecules 15.6 The monomer-polymer theme II: Biological macromolecules 15-2 The position of carbon in the periodic table Figure 15.1 15-3 The chemical diversity of organic compounds 4 carbons linked with single bonds, 1 oxygen and needed hydrogens. CH3 CH2 CH2 CH2 OH CH3 CH2 CH2 O CH3 CH3 CH2 CH CH3 OH CH3 CH3 CH CH2 OH CH3 O H2C CH2 H2C OH CH2 H2 C 15-4 H2C CH2 O CH CH2 CH2 CH3 O CH3 CH CH CH3 CH3 C CH3 OH CH2 CH CH3 CH O CH3 CH2 Figure 15.2 CH3 CH3 CH2 O CH2 CH3 H2 C CH CH2OH H2C CH CH2OH Figure 15.2 (continued) OH C H O CH2 CH CH2 CH3 H2 C CH CH3 O CH3 CH2 CH2 C O H2C C CH3 O H2C CH CH3 CH3 CH2 C CH3 O H CH3 CH2 O CH3 H2C CH3 CH C H O 15-5 H2C CH C H O CH2 C CH2 CH2 O C H2C CH CH3 HYDROCARBONS Carbon skeletons and hydrogen skins When determining the number of different skeletons, remember that: Each carbon forms a maximum of four single bonds, OR two single and one double bond, OR one single and triple bond. The arrangement of carbon atoms determines the skeleton, so a straight chain and a bent chain represent the same skeleton. Groups joined by single bonds can rotate, so a branch pointing down is the same as one pointing up. 15-6 Some five-carbon skeletons single bonds Figure 15.3 15-7 double bonds ring structures Adding a H-atom skin to a carbon skeleton Figure 15.4 15-8 SAMPLE PROBLEM 15.1 PROBLEM: Drawing hydrocarbons Draw structures that have different atom arrangements for hydrocarbons with: (a) six C atoms, no multiple bonds, and no rings (b) four C atoms, one double bond, and no rings (c) four C atoms, no multiple bonds, and one ring PLAN: Start with the longest chain and draw shorter chains until you are repeating structures. SOLUTION: (a) six carbons, no rings H H H H H H H H H H H H C C C C C C H H C C C C C H H H H H H H H H H H H C H H 15-9 H H H H H H C C C C C H H H H H H C H H SAMPLE PROBLEM 15.1 (continued) (a) continued: C C C C C C C C C C C C (b) four carbons, one double bond H H H H H H H C C C C H H C C C H H H H H H H H H C H H C C C C H H 15-10 H H (c) four carbons, one ring H H C H H C H H C H H C H C H C H H H C H C H H Table 15.1 Numerical Roots for Carbon Chains and Branches roots 15-11 number of carbon atoms meth- 1 eth- 2 prop- 3 but- 4 pent- 5 hex- 6 hept- 7 oct- 8 non- 9 dec- 10 15-12 Ways to depict formulas and models of an alkane Figure 15.5 15-13 Depicting cycloalkanes H H H C H C cyclopropane Figure 15.6 15-14 H C H H cyclobutane H H C C H H C C H H H Depicting cycloalkanes H H cyclopentane Figure 15.6 15-15 H H C H H C H C H C C H H C C H H H cyclohexane H H C H C H C H C H H H H 15-16 Boiling points of the first 10 unbranched alkanes Figure 15.7 15-17 An analogy for optical isomers Figure 15.8 15-18 Two chiral molecules optical isomers of 3-methylhexane Figure 15.9 15-19 optical isomers of alanine The rotation of plane-polarized light by an optically active substance Figure 15.10 15-20 The binding site of an enzyme Figure 15.11 15-21 15-22 The initial chemical event in vision Figure B15.1 15-23 SAMPLE PROBLEM 15.2 PROBLEM: Give the systematic name for each of the following, indicate the chiral center in part (d), and draw two geometric isomers for part (c). CH3 (a) Naming alkanes, alkenes and alkynes (b) CH3 C CH2 CH3 CH3 CH3 CH2 CH CH CH3 CH3 (d) (c) CH3 CH2 CH3 (e) CH3 CH3 CH2 CH CH2CH3 CH CH2 CH3 CH3 CH2 CH C CH CH3 CH3 PLAN: 15-24 For (a)-(c), find the longest, continuous chain and give it the base name (root + suffix). Then number the chain so that the branches occur on the lowest numbered carbons and name the branches with the (root + yl). For (d) and (e) the main chain must contain the double bond and the chain must be numbered such that the double bond occurs on the lowest numbered carbon. SAMPLE PROBLEM 15.2 (continued) methyl SOLUTION: methyl CH3 (a) CH3 (b) butane CH3 5CH2 4CH 3CH 6 CH3 2C CH CH3 3 2 1 2CH2 4 CH3 CH3 1 cyclopentane 3 4 5 2 1 CH3 methyl CH2CH3 ethyl 1-ethyl-2-methylcyclopentane methyl (d) pentene CH3 CH3 CH2 CH CH CH2 5 4 3 2 1 1-pentene 3-methyl-1-pentene chiral center 15-25 methyl hexane 3,4-dimethylhexane (can be numbered in either direction) methyl 2,2-dimethylbutane (c) CH3 SAMPLE PROBLEM 15.2 (continued) methyl CH3 (e) 6CH3 5CH2 CH C 4 3 3-hexene CH CH 1 3 2 CH3 methyl 3-hexene H CH3 C 6CH3 5CH2 methyl 4 C 3 CH CH 1 3 2 CH3 methyl cis-2,3-dimethyl-3-hexene 15-26 3-hexene methyl 6CH3 5CH2 4 H C CH3 C 3 CH CH 1 3 2 CH3 methyl trans-2,3-dimethyl-3-hexene Representations of benzene or Figure 15.12 15-27 Types of organic reactions An addition reaction occurs when an unsaturated reactant becomes a saturated product: X Y R CH CH R + X Y R CH CH R Elimination reactions are the opposite of addition; they occur when a more saturated reactant becomes a less saturated product: X Y R CH CH R R CH CH R + X Y A substitution reaction occurs when an atom (or group) from an added reagent substitutes for one in the organic reactant: R C X 15-28 + Y R C Y + X A color test for C=C bonds C C Figure 15.13 15-29 + Br2 Br C C Br 15-30 SAMPLE PROBLEM 15.3 PROBLEM: Recognizing the type of organic reaction State whether each reaction is an addition, elimination, or substitution: (a) CH3 CH2 CH2 Br (b) + H2 O (c) CH3 C Br + CH3CH2OH PLAN: O CH3 C OCH2CH3 + HBr Look for changes in the number of atoms attached to carbon. 15-31 CH3 CH CH2 + HBr More atoms bonded to carbon is an addition. Fewer atoms bonded to carbon is an elimination. Same number of atoms bonded to carbon is a substitution. SAMPLE PROBLEM 15.3 (continued) SOLUTION: (a) CH3 CH2 CH2 Br CH3 CH CH2 + HBr Elimination: there are fewer bonds to last two carbons. (b) + H2 Addition: there are more bonds to the two carbons in the second structure. O (c) CH3 C Br + CH3CH2OH O CH3 C OCH2CH3 + HBr Substitution: the C-Br bond becomes a C-O bond and the number of bonds to carbon remains the same. 15-32 Some molecules with the alcohol functional group Figure 15.14 15-33 15-34 15-35 General structures of amines the amine functional group primary (1o) amine Figure 15.15 15-36 secondary( 2o) amine C N tertiary (3o) amine Some biomolecules with the amine functional group lysine (1o amine) (amino acid found in proteins) epinephrine (adrenaline; 2o amine) (neurotransmitter in brain; hormone released during stress) Figure 15.16 15-37 adenine (1o amine) (component of nucleic acids) cocaine (3o amine) (brain stimulant; widely abused drug) Structure of a cationic detergent CH H3C N CH3 Cl H2C CH2 H2C CH2 benzylcetyldimethyl- H2C ammonium chloride H2C CH2 CH2 H2C CH2 H2C CH2 H2C CH2 Figure 15.17 H2C CH3 15-38 SAMPLE PROBLEM 15.4 PROBLEM: Predicting the reactions of alcohols, alkyl halides, and amines Determine the reaction type and predict the product(s) in the following: (a) CH3 CH2 CH2 I + NaOH (b) CH3 CH2 Br + 2 H3C CH2 CH2 NH2 Cr2O72- (c) H3C CH CH3 OH PLAN: 15-39 H2SO4 Check for functional groups and reagents, then for inorganics added. In (a) the -OH will substitute in the alkyl halide; in (b) the amine and alkyl halide will undergo a substitution of amine for halogen; in (c) the inorganics form a strong oxidizing agent resulting in an elimination. SAMPLE PROBLEM 15.4 (continued) SOLUTION: (a) substitution - the products are: CH3 CH2 CH2 OH + NaI (b) substitution - the products are: CH3 CH2 NHCH2CH2CH3 + CH3CH2CH2NHBr (c) elimination - the product is: H3C C CH3 O 15-40 Some common aldehydes and ketones methanal (formaldehyde) used to make resins in plywood, dishware, countertops; biological preservative ethanal (acetaldehyde) narcotic product of ethanol metabolism; used to make perfume, flavors, plastics, other chemicals 2-propanone benzaldehyde (acetone) solvent artificial for fat, rubber, almond plastic, varnish, flavoring lacquer; chemical feedstock 2-butanone (methyl ethyl ketone) important solvent Figure 15.18 15-41 The carbonyl group Figure 15.19 15-42 SAMPLE PROBLEM 15.5 PROBLEM: Predicting the steps in a reaction sequence Fill in the blanks in the following reaction sequence: Br OH- CH3 CH2 CH CH3 Cr2O72- CH3Li H2O H2SO4 PLAN: Look at the functional groups and reagents to determine the type of reaction. SOLUTION: Br OH- OH O Cr2 O7 2- CH3 CH2 CH CH3 CH3 CH2 CH CH3 2-bromobutane 2-butanol CH3 CH2 C H2 SO4 2-butanone OH CH3 CH2 C CH3 Li CH3 CH3 2-methyl-2-butanol 15-43 CH3 H2 O Some molecules with the carboxylic acid functional group methanoic acid (formic acid) (an irritating component of ant and bee stings) benzoic acid (calorimetric standard; used in preserving food, dyeing fabric, curing tobacco) Figure 15.20 15-44 butanoic acid (butyric acid) (odor of rancid butter; suspected component of monkey sex attractant) octadecanoic acid (stearic acid) (found in animal fats; used in making candles and soap) Some lipid molecules with the ester functional group cetyl palmitate (the most common lipid in whale blubber) lecithin (phospholipid found in all cell membranes) 15-45 Figure 15.21 tristearin (typical dietary fat used as an energy store in animals) Which reactant contributes which group to the ester? General esterification reaction O R C OH + + H OR' 18 R C OH + R' OH 18 R C OH + R' 18 R C O R' + H O H O O 15-46 R C O R' + H O H O O Figure 15.22 H O 18 OH R C O R' + H O H Some molecules with the amide functional group acetaminophen (active ingredient in nonaspirin pain relievers; used to make dyes and photographic chemicals) N,N-dimethylmethanamide (dimethylformamide) (major organic solvent; used in production of synthetic fibers) Figure 15.23 15-47 lysergic acid diethylamide (LSD-25) (a potent hallucinogen) SAMPLE PROBLEM 15.6 PROBLEM: Predicting the reactions of the carboxylic acid family Predict the product(s) of the following reactions: O OH (a) CH3 CH2 CH2 C OH CH3 + CH3 CH CH3 O (b) CH3 CH CH2 CH2 C NH CH2 CH3 PLAN: H+ NaOH H2O (a) An acid and an alcohol undergo a condensation reaction to form an ester. (b) An amide, in the presence of base and water, is hydrolyzed. O CH3 SOLUTION: (a) CH3 CH2 CH2 C O CH CH3 CH3 O (b) CH3 CH CH2 CH2 C O- + Na+ + NH2 CH2 CH3 15-48 The formation of carboxylic, phosphoric, and sulfuric acid anhydrides Figure 15.24 15-49 An ester and an amide of other non-metals glucose-6-phosphate Figure 15.25 15-50 sulfanilamide SAMPLE PROBLEM 15.7 PROBLEM: Circle and name the functional groups in the following molecules: O (a) Recognizing functional groups C OH O O (c) (b) OH O C CH3 CH CH2 NH CH3 Cl PLAN: Use Table 15.5 to identify the functional groups. SOLUTION: carboxylic O acid (a) C OH O O C CH3 ketone (b) (c) alcohol OH O haloalkane CH CH2 NH CH3 Cl ester 2o amine alkene 15-51 A summary of the interconversions among the major organic functional groups Figure 15.26 15-52 Steps in the free-radical polymerization of ethylene Figure 15.27 15-53 15-54 15-55 The formation of nylon-66 Figure 15.28 15-56 The structure of glucose in aqueous solution and the formation of a disaccharide 15-57 Figure 15.29 The common amino acids 15-58 Figure 15.30 A portion of a polypeptide chain Figure 15.31 15-59 Forces that maintain protein structure Figure 15.32 15-60 Shapes of fibrous proteins Figure 15.33 collagen 15-61 silk fibroin Mononucleotide monomers and their linkage portion of DNA polynucleotide chain mononucleotide of ribonucleic acid (RNA) Figure 15.34 15-62 mononucleotide of deoxyribonucleic acid (DNA) The double helix of DNA 1.08 nm Figure 15.35 15-63 1.08 nm Key stages in protein synthesis Figure 15.36 15-64 Key stages of DNA replication 15-65 Figure 15.37